Professional Documents
Culture Documents
IWA 29TH PKM TRG 2021.12.01 08.35.17 Details
Uploaded by
akreditasi tarogong 2023Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
IWA 29TH PKM TRG 2021.12.01 08.35.17 Details
Uploaded by
akreditasi tarogong 2023Copyright:
Available Formats
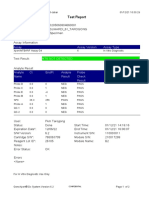
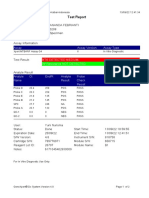
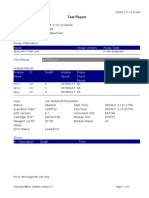
110003011-PKM Tarogong-Garut-Jabar 01/12/21 10:50:10
Test Report
Patient ID:
Sample ID: IWA 29TH PKM TRG
Test Type: Specimen
Sample Type:
Assay Information
Assay Assay Version Assay Type
Xpert MTB-RIF Assay G4 6 In Vitro Diagnostic
Test Result: MTB NOT DETECTED
-
Analyte Result
Analyte Ct EndPt Analyte Probe
Name Result Check
Result
Probe D 0.0 1 NEG PASS
Probe C 0.0 -5 NEG PASS
Probe E 0.0 -2 NEG PASS
Probe B 0.0 1 NEG PASS
SPC 29.6 237 PASS PASS
Probe A 0.0 0 NEG PASS
QC-1 0.0 0 NEG PASS
QC-2 0.0 0 NEG PASS
User: Pkm Tarogong
Status: Done Start Time: 01/12/21 08:35:17
Expiration Date*: 12/06/22 End Time: 01/12/21 10:17:07
S/W Version: 6.2 Instrument S/N: 110003011
Cartridge S/N*: 765064544 Module S/N: 210017267
Reagent Lot ID*: 26105 Module Name: B1
Notes:
Error Status: OK
-
Errors
<None>
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 6.2 CONFIDENTIAL Page 1 of 4
110003011-PKM Tarogong-Garut-Jabar 01/12/21 10:50:10
Test Report
Tech. Initial/Date Supervisor Initial/Date
* indicates that a particular field is entered using a barcode scanner
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 6.2 CONFIDENTIAL Page 2 of 4
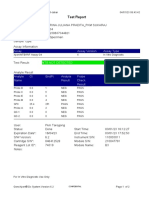
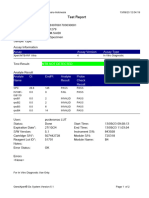
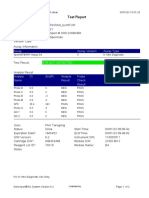
110003011-PKM Tarogong-Garut-Jabar 01/12/21 10:50:10
Test Report
Patient ID:
Sample ID: AHMAD AMARILIS 3
Test Type: Specimen
Sample Type:
Assay Information
Assay Assay Version Assay Type
Xpert MTB-RIF Assay G4 6 In Vitro Diagnostic
Test Result: MTB NOT DETECTED
-
Analyte Result
Analyte Ct EndPt Analyte Probe
Name Result Check
Result
Probe D 0.0 1 NEG PASS
Probe C 0.0 -5 NEG PASS
Probe E 0.0 -2 NEG PASS
Probe B 0.0 1 NEG PASS
SPC 25.2 247 PASS PASS
Probe A 0.0 1 NEG PASS
QC-1 0.0 0 NEG PASS
QC-2 0.0 0 NEG PASS
User: Pkm Tarogong
Status: Done Start Time: 30/11/21 11:00:01
Expiration Date*: 12/06/22 End Time: 30/11/21 12:42:06
S/W Version: 6.2 Instrument S/N: 110003011
Cartridge S/N*: 765064531 Module S/N: 210017267
Reagent Lot ID*: 26105 Module Name: B1
Notes:
Error Status: OK
-
Errors
<None>
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 6.2 CONFIDENTIAL Page 3 of 4
110003011-PKM Tarogong-Garut-Jabar 01/12/21 10:50:10
Test Report
Tech. Initial/Date Supervisor Initial/Date
* indicates that a particular field is entered using a barcode scanner
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 6.2 CONFIDENTIAL Page 4 of 4
You might also like
- Digital Circuit Testing: A Guide to DFT and Other TechniquesFrom EverandDigital Circuit Testing: A Guide to DFT and Other TechniquesNo ratings yet
- NANI K 46TH PASUNDAN 2021.12.01 08.36.02 DetailsDocument4 pagesNANI K 46TH PASUNDAN 2021.12.01 08.36.02 Detailsakreditasi tarogong 2023No ratings yet
- Transistor Electronics: Use of Semiconductor Components in Switching OperationsFrom EverandTransistor Electronics: Use of Semiconductor Components in Switching OperationsRating: 1 out of 5 stars1/5 (1)
- SUHARDI 61 TAROGONG 2021.12.01 14.18.16 DetailsDocument2 pagesSUHARDI 61 TAROGONG 2021.12.01 14.18.16 Detailsakreditasi tarogong 2023No ratings yet
- Practical Data Acquisition for Instrumentation and Control SystemsFrom EverandPractical Data Acquisition for Instrumentation and Control SystemsNo ratings yet
- APIHDocument2 pagesAPIHpuskesmas cigeulisNo ratings yet
- 2023.01.03 08.06.58 DetailsDocument2 pages2023.01.03 08.06.58 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 16.12.27 DetailsDocument2 pages2023.01.03 16.12.27 Detailsakreditasi tarogong 2023No ratings yet
- 2023.05.06 08.22.15 DetailsDocument2 pages2023.05.06 08.22.15 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 15.25.33 DetailsDocument2 pages2023.01.03 15.25.33 Detailsakreditasi tarogong 2023No ratings yet
- Tamron IDocument2 pagesTamron IEri OiNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay Typeyossy aprillyaNo ratings yet
- Cihideung TCM DetailsDocument10 pagesCihideung TCM Detailsmulyadi diningrumNo ratings yet
- 2023.01.03 11.48.53 DetailsDocument2 pages2023.01.03 11.48.53 Detailsakreditasi tarogong 2023No ratings yet
- PKM BetoambariDocument6 pagesPKM Betoambarinur liylaNo ratings yet
- 2023.01.03 09.59.10 DetailsDocument2 pages2023.01.03 09.59.10 Detailsakreditasi tarogong 2023No ratings yet
- SUKARMINI-DPM DR ABDUL GHOFIRDocument2 pagesSUKARMINI-DPM DR ABDUL GHOFIRlaboratorium mitra utamaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument6 pagesTest Report: Assay Assay Version Assay TypeJose F. Ramirez MendozaNo ratings yet
- Bungga Hobrouw (R.anak)Document2 pagesBungga Hobrouw (R.anak)lukas mansnandifuNo ratings yet
- Nurbaya 2023.02.02 14.49.06 DetailsDocument2 pagesNurbaya 2023.02.02 14.49.06 DetailsRahmatul LailiNo ratings yet
- Hasim. S1Document2 pagesHasim. S1pkmsilo1No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypesetyawanankesNo ratings yet
- Ananda FebriantiDocument2 pagesAnanda FebriantiInternis RsuyarsiNo ratings yet
- 3Document6 pages3Jose F. Ramirez MendozaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypeMelania Dessy Savitri SunarjadiNo ratings yet
- Rsi Sa 12-10-2023Document6 pagesRsi Sa 12-10-2023Laboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- M.SAIDI 2023.09.13 09.58.13 DetailsDocument2 pagesM.SAIDI 2023.09.13 09.58.13 DetailsLaboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- Instrum 301122144638 2022.11.30 14.47.40 DetailsDocument1 pageInstrum 301122144638 2022.11.30 14.47.40 DetailsLabovida RoraimaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument3 pagesTest Report: Assay Assay Version Assay TypeAlex MoralesNo ratings yet
- Qa Test Format X RayDocument9 pagesQa Test Format X RayNIKHIL VERMANo ratings yet
- Variant Ii Turbo Hemoglobin Testing System: Touseasanaidin The Diagnosis of DiabetesDocument6 pagesVariant Ii Turbo Hemoglobin Testing System: Touseasanaidin The Diagnosis of DiabetesKetevan MigriauliNo ratings yet
- Alba Report - CompressedDocument30 pagesAlba Report - Compressedwinston11No ratings yet
- PCRDocument6 pagesPCRJose F. Ramirez MendozaNo ratings yet
- VARGA ResultsDocument3 pagesVARGA ResultsCocos MirelNo ratings yet
- Variant Ii Hemoglobin Testing System For Hba: Bio-Rad LaboratoriesDocument6 pagesVariant Ii Hemoglobin Testing System For Hba: Bio-Rad LaboratoriesBhageshwar ChaudharyNo ratings yet
- Package Qualification Report: Reliability by DesignDocument2 pagesPackage Qualification Report: Reliability by DesignAnthony C.No ratings yet
- OVP - OQ Test Protocol: Overall Test Result PASSEDDocument8 pagesOVP - OQ Test Protocol: Overall Test Result PASSEDAli RizviNo ratings yet
- 6MD61xx KEMA-Certificate IEC61850 V4Document2 pages6MD61xx KEMA-Certificate IEC61850 V4Nguyễn Tuấn ViệtNo ratings yet
- 31-03-2020 C5 HRSG - LP Reedwater To HRSG DRUM Level Control Valve - PTDocument3 pages31-03-2020 C5 HRSG - LP Reedwater To HRSG DRUM Level Control Valve - PTDave CheungNo ratings yet
- Reg0012 - KVP Accuracy: 1 Personnel RequirementsDocument66 pagesReg0012 - KVP Accuracy: 1 Personnel RequirementsGeorgiana KokonaNo ratings yet
- CertificateofAnalysis 2021 12 1 971796Document2 pagesCertificateofAnalysis 2021 12 1 971796adiazcalidadNo ratings yet
- Meghna PVC LTD (Pet Division) QAC Department Shift Chemist Log BookDocument5 pagesMeghna PVC LTD (Pet Division) QAC Department Shift Chemist Log Bookswapon kumar shillNo ratings yet
- Wavelength accuracy test passesDocument2 pagesWavelength accuracy test passescarlos germanNo ratings yet
- Calibration ProcedureDocument12 pagesCalibration ProcedureMuhammadSadeliAmliNo ratings yet
- Certificate of Relief Valve Capacity (PROCA) 7 PDFDocument1 pageCertificate of Relief Valve Capacity (PROCA) 7 PDFAdeoye OkunoyeNo ratings yet
- LabUPlus SerialconnectDocument7 pagesLabUPlus SerialconnectJose Perez PerezNo ratings yet
- 11-03-2020 C1 Gland Steam Super Heater - UTDocument4 pages11-03-2020 C1 Gland Steam Super Heater - UTDave CheungNo ratings yet
- 10-1982 SJM Certificate PCS-900Document2 pages10-1982 SJM Certificate PCS-900anon_238578985No ratings yet
- PQ Test Protocol MPA Sphere 20231211 212421Document5 pagesPQ Test Protocol MPA Sphere 20231211 212421Ali RizviNo ratings yet
- MIghty Machine RejectDocument1 pageMIghty Machine RejectMohammad Shanawaz MNo ratings yet
- Client: Pt. STBC Location: Autoclave 3 & 4 Report No.: 001-PAUT/RBT-STBC/XI/2022Document60 pagesClient: Pt. STBC Location: Autoclave 3 & 4 Report No.: 001-PAUT/RBT-STBC/XI/2022Rizal HidayatullahNo ratings yet
- Worksheet - PTDocument5 pagesWorksheet - PTDave CheungNo ratings yet
- 22 2806 DNV Thytronic XMR P XMR A XMR D XMR T XMR V Server Ed2 Certificate Be33f777f6Document2 pages22 2806 DNV Thytronic XMR P XMR A XMR D XMR T XMR V Server Ed2 Certificate Be33f777f6Chimaroke UmunnaNo ratings yet
- PQ Test Protocol MPA Sample Compartment 20231211 222536Document5 pagesPQ Test Protocol MPA Sample Compartment 20231211 222536Ali RizviNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument7 pagesTest Report: Assay Assay Version Assay TyperosmayaniimutzNo ratings yet
- Unit-1 10KV 10BBB01 P127Document41 pagesUnit-1 10KV 10BBB01 P127MühâMméd SàhãdhNo ratings yet
- INST-JTBACNET Fantech CarparkDocument16 pagesINST-JTBACNET Fantech CarparkInventor SolidworksNo ratings yet
- Ludlum Model 15 Neutron Counter ManualDocument44 pagesLudlum Model 15 Neutron Counter Manualsamel abreNo ratings yet
- Instruction For C-Reactive Protein (CRP) Detection Kit (Nephelometry)Document2 pagesInstruction For C-Reactive Protein (CRP) Detection Kit (Nephelometry)Muhammad KhalidNo ratings yet
- Certificado Test ReportDocument28 pagesCertificado Test ReportdronebessaNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- Xpert M 030123080450 2023.01.03 08.05.42 DetailsDocument2 pagesXpert M 030123080450 2023.01.03 08.05.42 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 15.25.33 DetailsDocument2 pages2023.01.03 15.25.33 Detailsakreditasi tarogong 2023No ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- 2023.01.03 11.48.53 DetailsDocument2 pages2023.01.03 11.48.53 Detailsakreditasi tarogong 2023No ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- Sex, Gender and Health BiotechnologyDocument9 pagesSex, Gender and Health BiotechnologymoonchildNo ratings yet
- Nidhi-Tbi: National Initiative For Developing and Harnessing InnovationsDocument52 pagesNidhi-Tbi: National Initiative For Developing and Harnessing InnovationsshashiNo ratings yet
- NanoparticlesDocument29 pagesNanoparticlesMOHAMMAD ARSHAD PATHANNo ratings yet
- PRISM SOP Supplemental MaterialsDocument7 pagesPRISM SOP Supplemental MaterialsJaya TomNo ratings yet
- Cell - Structure and Function: Module - 1Document35 pagesCell - Structure and Function: Module - 1Manas TripathiNo ratings yet
- The Structure and Function of MacromoleculesDocument50 pagesThe Structure and Function of MacromoleculesVeronica P. CapoteNo ratings yet
- The OneDocument436 pagesThe OneSheena ChenNo ratings yet
- Admin,+Journal+Manager,+12 AJPCR 40 31720Document3 pagesAdmin,+Journal+Manager,+12 AJPCR 40 31720Komal RaneNo ratings yet
- M.Sc. Biosciences Department ProfileDocument20 pagesM.Sc. Biosciences Department ProfileRahul MohantyNo ratings yet
- Prof. Dr. David Buntoro Drg. MDS. SP-BMKDocument30 pagesProf. Dr. David Buntoro Drg. MDS. SP-BMKDipdha Arum SangoraNo ratings yet
- Cancer As A Mitochondrial Metabolic Disease: Thomas N. SeyfriedDocument12 pagesCancer As A Mitochondrial Metabolic Disease: Thomas N. SeyfriedCatherine BulsecoNo ratings yet
- CTAB ModificadoDocument6 pagesCTAB ModificadoDILERY AHTZIRY JUAREZ MONROYNo ratings yet
- CRISPR/Cas genome editing of human 3PN embryosDocument2 pagesCRISPR/Cas genome editing of human 3PN embryosMaría Clara BoteroNo ratings yet
- Discussion Bio460Document2 pagesDiscussion Bio460MUHAMMAD SYAHMI BIN AZMINo ratings yet
- G10 Science Q3 - Week 4 - MutationDocument48 pagesG10 Science Q3 - Week 4 - Mutationmaclarrisse.biacoNo ratings yet
- 8.3 DNA Worksheet StudentDocument4 pages8.3 DNA Worksheet StudentVippo Montecillo0% (1)
- Nitrogenous Bases, Nucleotides and Nucleic Acids - For UploadDocument30 pagesNitrogenous Bases, Nucleotides and Nucleic Acids - For UploadJ dawgNo ratings yet
- Drugs For Chemical EngineeringDocument34 pagesDrugs For Chemical Engineeringshivakumar hrNo ratings yet
- Vaccine Schedule Recommended Adults 2022 v03Document2 pagesVaccine Schedule Recommended Adults 2022 v03mariumNo ratings yet
- Five Major Steps of Paraffin Embedding - ImmunostainingDocument7 pagesFive Major Steps of Paraffin Embedding - ImmunostainingImmunostainingNo ratings yet
- VACCINES COVID-19 INVIGORATES A STAGNANT INDUSTRYresDocument22 pagesVACCINES COVID-19 INVIGORATES A STAGNANT INDUSTRYresiyad.alsabiNo ratings yet
- Vaccine Information For 11-Year-Old StudentsDocument1 pageVaccine Information For 11-Year-Old StudentsAnonymous gH0Y8V0No ratings yet
- Plant Biochemical Defense MechanismDocument18 pagesPlant Biochemical Defense MechanismMelodramatic FoolNo ratings yet
- Getting The Picture From DNADocument3 pagesGetting The Picture From DNANguyễn Phạm Thảo Nguyên0% (3)
- 8 3 PhotosynthesisDocument39 pages8 3 PhotosynthesisKhin (Darin) Hnin PhyuNo ratings yet
- Presented By:-Shubham Nayak 12111044 BiotechnologyDocument19 pagesPresented By:-Shubham Nayak 12111044 BiotechnologyMukul SuryawanshiNo ratings yet
- Cumulative Lab ReportDocument3 pagesCumulative Lab ReportaplesgjskNo ratings yet
- CHAPTER 12 Protein SynthesisDocument10 pagesCHAPTER 12 Protein SynthesisNadeem IqbalNo ratings yet
- Admissio Ns Guide Admissio Ns Guide: For O/N (A) - Level S TudentsDocument38 pagesAdmissio Ns Guide Admissio Ns Guide: For O/N (A) - Level S TudentsweiweiahNo ratings yet
- Barriers of Protein and Peptide Drug DeliveryDocument12 pagesBarriers of Protein and Peptide Drug DeliveryAashish chaudhari100% (2)