Professional Documents
Culture Documents
Nurbaya 2023.02.02 14.49.06 Details
Uploaded by
Rahmatul LailiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Nurbaya 2023.02.02 14.49.06 Details
Uploaded by
Rahmatul LailiCopyright:
Available Formats
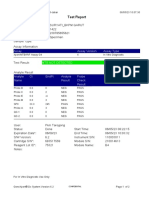
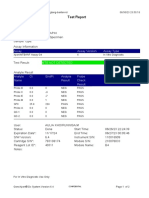
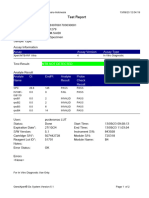
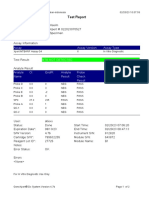
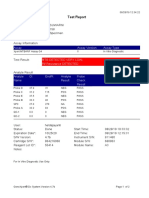
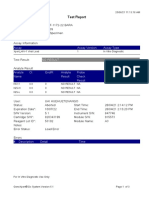
819108-PUSKESMAS WOHA-NTB 06/02/23 10:55:30
Test Report
Patient ID: 5206115106500001
Sample ID: Nurbaya
Test Type: Specimen
Sample Type:
Assay Information
Assay Assay Version Assay Type
Xpert MTB-RIF Assay G4 6 In Vitro Diagnostic
Test Result: MTB DETECTED HIGH;
Rif Resistance NOT DETECTED
-
Analyte Result
Analyte Ct EndPt Analyte Probe
Name Result Check
Result
Probe D 10.6 262 POS PASS
Probe C 10.7 218 POS PASS
Probe E 12.6 73 POS PASS
Probe B 11.5 106 POS PASS
SPC 23.8 266 NA PASS
Probe A 10.3 84 POS PASS
QC-1 0.0 0 NEG PASS
QC-2 0.0 0 NEG PASS
User: puskesmas
Status: Done Start Time: 02/02/23 14:49:06
Expiration Date*: 10/11/24 End Time: 02/02/23 16:31:25
S/W Version: 4.8 Instrument S/N: 819108
Cartridge S/N*: 778790433 Module S/N: 715634
Reagent Lot ID*: 40305 Module Name: C4
Notes: salifa
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 4.8 Page 1 of 2
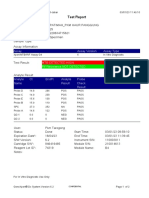
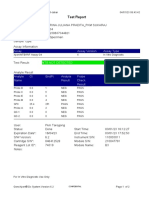
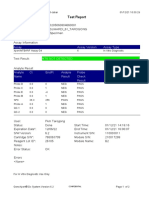
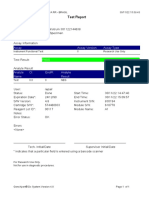
819108-PUSKESMAS WOHA-NTB 06/02/23 10:55:30
Test Report
Error Status: OK
-
Errors
<None>
Tech. Initial/Date Supervisor Initial/Date
* indicates that a particular field is entered using a barcode scanner
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 4.8 Page 2 of 2
You might also like
- Cihideung TCM DetailsDocument10 pagesCihideung TCM Detailsmulyadi diningrumNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay Typeyossy aprillyaNo ratings yet
- 3Document6 pages3Jose F. Ramirez MendozaNo ratings yet
- Bungga Hobrouw (R.anak)Document2 pagesBungga Hobrouw (R.anak)lukas mansnandifuNo ratings yet
- 2023.05.06 08.22.15 DetailsDocument2 pages2023.05.06 08.22.15 Detailsakreditasi tarogong 2023No ratings yet
- PKM BetoambariDocument6 pagesPKM Betoambarinur liylaNo ratings yet
- 2023.01.03 08.06.58 DetailsDocument2 pages2023.01.03 08.06.58 Detailsakreditasi tarogong 2023No ratings yet
- SUKARMINI-DPM DR ABDUL GHOFIRDocument2 pagesSUKARMINI-DPM DR ABDUL GHOFIRlaboratorium mitra utamaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument6 pagesTest Report: Assay Assay Version Assay TypeJose F. Ramirez MendozaNo ratings yet
- APIHDocument2 pagesAPIHpuskesmas cigeulisNo ratings yet
- Rsi Sa 12-10-2023Document6 pagesRsi Sa 12-10-2023Laboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- Ananda FebriantiDocument2 pagesAnanda FebriantiInternis RsuyarsiNo ratings yet
- IWA 29TH PKM TRG 2021.12.01 08.35.17 DetailsDocument4 pagesIWA 29TH PKM TRG 2021.12.01 08.35.17 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 15.25.33 DetailsDocument2 pages2023.01.03 15.25.33 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 09.59.10 DetailsDocument2 pages2023.01.03 09.59.10 Detailsakreditasi tarogong 2023No ratings yet
- NANI K 46TH PASUNDAN 2021.12.01 08.36.02 DetailsDocument4 pagesNANI K 46TH PASUNDAN 2021.12.01 08.36.02 Detailsakreditasi tarogong 2023No ratings yet
- M.SAIDI 2023.09.13 09.58.13 DetailsDocument2 pagesM.SAIDI 2023.09.13 09.58.13 DetailsLaboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- Hasim. S1Document2 pagesHasim. S1pkmsilo1No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypesetyawanankesNo ratings yet
- 2023.01.03 16.12.27 DetailsDocument2 pages2023.01.03 16.12.27 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 11.48.53 DetailsDocument2 pages2023.01.03 11.48.53 Detailsakreditasi tarogong 2023No ratings yet
- Tamron IDocument2 pagesTamron IEri OiNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypeMelania Dessy Savitri SunarjadiNo ratings yet
- SUHARDI 61 TAROGONG 2021.12.01 14.18.16 DetailsDocument2 pagesSUHARDI 61 TAROGONG 2021.12.01 14.18.16 Detailsakreditasi tarogong 2023No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument3 pagesTest Report: Assay Assay Version Assay TypeAlex MoralesNo ratings yet
- Instrum 301122144638 2022.11.30 14.47.40 DetailsDocument1 pageInstrum 301122144638 2022.11.30 14.47.40 DetailsLabovida RoraimaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument7 pagesTest Report: Assay Assay Version Assay TyperosmayaniimutzNo ratings yet
- PCRDocument6 pagesPCRJose F. Ramirez MendozaNo ratings yet
- Qa Test Format X RayDocument9 pagesQa Test Format X RayNIKHIL VERMANo ratings yet
- Variant Ii Turbo Hemoglobin Testing System: Touseasanaidin The Diagnosis of DiabetesDocument6 pagesVariant Ii Turbo Hemoglobin Testing System: Touseasanaidin The Diagnosis of DiabetesKetevan MigriauliNo ratings yet
- 0001ba008448 1 F1Document1 page0001ba008448 1 F1Ankush SainiNo ratings yet
- Client: Pt. STBC Location: Autoclave 3 & 4 Report No.: 001-PAUT/RBT-STBC/XI/2022Document60 pagesClient: Pt. STBC Location: Autoclave 3 & 4 Report No.: 001-PAUT/RBT-STBC/XI/2022Rizal HidayatullahNo ratings yet
- VARGA ResultsDocument3 pagesVARGA ResultsCocos MirelNo ratings yet
- DocUReader 2 Operators ManualDocument85 pagesDocUReader 2 Operators ManualKinnari BhattNo ratings yet
- Variant Ii Hemoglobin Testing System For Hba: Bio-Rad LaboratoriesDocument6 pagesVariant Ii Hemoglobin Testing System For Hba: Bio-Rad LaboratoriesBhageshwar ChaudharyNo ratings yet
- DocUReader 2 PRO Uputstvo enDocument66 pagesDocUReader 2 PRO Uputstvo enDarko MaksimovicNo ratings yet
- CT100B System User ManualDocument22 pagesCT100B System User ManualJose RojasNo ratings yet
- P L Sharma District Hospital Meerut SHF 835Document5 pagesP L Sharma District Hospital Meerut SHF 835rajeshk.praja1997No ratings yet
- RNA Clean 17-8 - Eukaryote Total RNA Nano - DE72905431 - 2022-08-18 - 11-23-22Document15 pagesRNA Clean 17-8 - Eukaryote Total RNA Nano - DE72905431 - 2022-08-18 - 11-23-22Sara SolimanNo ratings yet
- Fit-2200 - Daenerys 1HDocument4 pagesFit-2200 - Daenerys 1HCarlos Salazar GarciaNo ratings yet
- Plot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Document3 pagesPlot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Asit ANo ratings yet
- Erba Laura M User ManualDocument30 pagesErba Laura M User ManualDaniel VargasNo ratings yet
- OM ClotQuant 4Document28 pagesOM ClotQuant 4Jose PersiaNo ratings yet
- Asset 1731 QA ReportDocument5 pagesAsset 1731 QA Reportrubyhall bio-medicalNo ratings yet
- Tuv Report: Sample InformationDocument1 pageTuv Report: Sample InformationBiotomy LifesciencesNo ratings yet
- SOP For ÄKTA Pure Chromatography System OperationDocument20 pagesSOP For ÄKTA Pure Chromatography System Operation王仁宏No ratings yet
- Kolkata: Calibration: Ofcalibration: IDocument4 pagesKolkata: Calibration: Ofcalibration: IjamilNo ratings yet
- Niraj Agrawal - 27Document3 pagesNiraj Agrawal - 27Ankit pattnaikNo ratings yet
- 6MD61xx KEMA-Certificate IEC61850 V4Document2 pages6MD61xx KEMA-Certificate IEC61850 V4Nguyễn Tuấn ViệtNo ratings yet
- PF2P5MKX-2023-09-18-231029Document16 pagesPF2P5MKX-2023-09-18-231029tikagenouNo ratings yet
- Ultrasonic Exam Report Steam Drum JointsDocument3 pagesUltrasonic Exam Report Steam Drum JointsBalkishan DyavanapellyNo ratings yet
- A-Star Testing & Inspection (S) Pte LTD: Magnetic Particle Testing ReportDocument4 pagesA-Star Testing & Inspection (S) Pte LTD: Magnetic Particle Testing ReportHari KarthickNo ratings yet
- 10-1982 SJM Certificate PCS-900Document2 pages10-1982 SJM Certificate PCS-900anon_238578985No ratings yet
- COVID-19 Test Report for Ms. MAYA DEVKOTADocument1 pageCOVID-19 Test Report for Ms. MAYA DEVKOTARoshan RayNo ratings yet
- MIghty Machine RejectDocument1 pageMIghty Machine RejectMohammad Shanawaz MNo ratings yet
- SAR Test ReportDocument81 pagesSAR Test ReportEngenheiro Stefan ObermarkNo ratings yet
- NM002C23421596794477 - RLS 3 Converted 2Document3 pagesNM002C23421596794477 - RLS 3 Converted 2pravins93No ratings yet
- Jytszb-R12-2100610 en 300328 WifiDocument77 pagesJytszb-R12-2100610 en 300328 WifionallpelinNo ratings yet
- 23 11 21 ExtractProtocolJoan GoodLadderDocument18 pages23 11 21 ExtractProtocolJoan GoodLadderle.gonzalez2075No ratings yet