Professional Documents
Culture Documents
SUKARMINI-DPM DR ABDUL GHOFIR
Uploaded by
laboratorium mitra utama0 ratings0% found this document useful (0 votes)
1 views2 pagesOriginal Title
SUKARMINI-DPM dr ABDUL GHOFIR
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
1 views2 pagesSUKARMINI-DPM DR ABDUL GHOFIR
Uploaded by
laboratorium mitra utamaCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
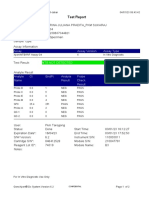
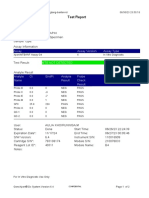
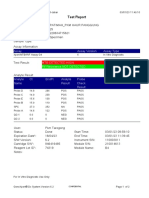
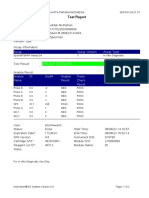
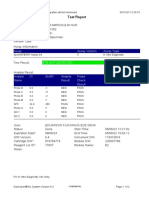
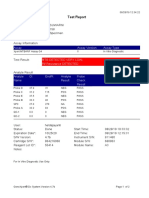
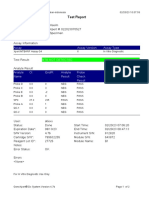
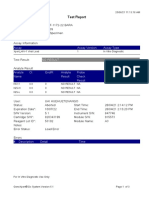
844132_RSUD JOMBANG-JAWA TIMUR-INDONESIA 09/02/23 07:40:57
Test Report
Patient ID: SUKARMINI-DPM dr ABDUL GHOFIR
Patient ID 2: 2404 J-23
Sample ID*: 230950380721
Test Type: Specimen
Sample Type:
Assay Information
Assay Assay Version Assay Type
Xpert MTB-RIF Assay G4 6 In Vitro Diagnostic
Test Result: MTB DETECTED VERY LOW;
Rif Resistance NOT DETECTED
-
Analyte Result
Analyte Ct EndPt Analyte Probe
Name Result Check
Result
Probe D 30.1 123 POS PASS
Probe C 29.3 110 POS PASS
Probe E 31.8 96 POS PASS
Probe B 29.1 104 POS PASS
SPC 25.3 255 NA PASS
Probe A 29.6 79 POS PASS
QC-1 0.0 0 NEG PASS
QC-2 0.0 0 NEG PASS
User: Danang Tri PS
Status: Done Start Time: 09/01/23 19:38:54
Expiration Date*: 09/01/24 End Time: 09/01/23 21:19:58
S/W Version: 5.1 Instrument S/N: 740056
Cartridge S/N*: 417031465 Module S/N: 617465
Reagent Lot ID*: 29712 Module Name: B4
Notes:
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 5.1 Page 1 of 2
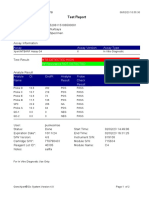
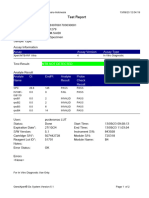
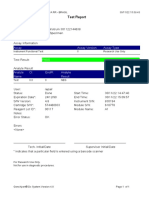
844132_RSUD JOMBANG-JAWA TIMUR-INDONESIA 09/02/23 07:40:57
Test Report
Error Status: OK
-
Errors
<None>
Tech. Initial/Date Supervisor Initial/Date
* indicates that a particular field is entered using a barcode scanner
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 5.1 Page 2 of 2
You might also like
- 2023.01.03 11.48.53 DetailsDocument2 pages2023.01.03 11.48.53 Detailsakreditasi tarogong 2023No ratings yet
- Testing UMTS: Assuring Conformance and Quality of UMTS User EquipmentFrom EverandTesting UMTS: Assuring Conformance and Quality of UMTS User EquipmentNo ratings yet
- 2023.01.03 15.25.33 DetailsDocument2 pages2023.01.03 15.25.33 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 16.12.27 DetailsDocument2 pages2023.01.03 16.12.27 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 08.06.58 DetailsDocument2 pages2023.01.03 08.06.58 Detailsakreditasi tarogong 2023No ratings yet
- Cihideung TCM DetailsDocument10 pagesCihideung TCM Detailsmulyadi diningrumNo ratings yet
- 2023.05.06 08.22.15 DetailsDocument2 pages2023.05.06 08.22.15 Detailsakreditasi tarogong 2023No ratings yet
- IWA 29TH PKM TRG 2021.12.01 08.35.17 DetailsDocument4 pagesIWA 29TH PKM TRG 2021.12.01 08.35.17 Detailsakreditasi tarogong 2023No ratings yet
- APIHDocument2 pagesAPIHpuskesmas cigeulisNo ratings yet
- Nurbaya 2023.02.02 14.49.06 DetailsDocument2 pagesNurbaya 2023.02.02 14.49.06 DetailsRahmatul LailiNo ratings yet
- 3Document6 pages3Jose F. Ramirez MendozaNo ratings yet
- 2023.01.03 09.59.10 DetailsDocument2 pages2023.01.03 09.59.10 Detailsakreditasi tarogong 2023No ratings yet
- NANI K 46TH PASUNDAN 2021.12.01 08.36.02 DetailsDocument4 pagesNANI K 46TH PASUNDAN 2021.12.01 08.36.02 Detailsakreditasi tarogong 2023No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay Typeyossy aprillyaNo ratings yet
- PKM BetoambariDocument6 pagesPKM Betoambarinur liylaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument6 pagesTest Report: Assay Assay Version Assay TypeJose F. Ramirez MendozaNo ratings yet
- Bungga Hobrouw (R.anak)Document2 pagesBungga Hobrouw (R.anak)lukas mansnandifuNo ratings yet
- SUHARDI 61 TAROGONG 2021.12.01 14.18.16 DetailsDocument2 pagesSUHARDI 61 TAROGONG 2021.12.01 14.18.16 Detailsakreditasi tarogong 2023No ratings yet
- Tamron IDocument2 pagesTamron IEri OiNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypeMelania Dessy Savitri SunarjadiNo ratings yet
- Rsi Sa 12-10-2023Document6 pagesRsi Sa 12-10-2023Laboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- Ananda FebriantiDocument2 pagesAnanda FebriantiInternis RsuyarsiNo ratings yet
- M.SAIDI 2023.09.13 09.58.13 DetailsDocument2 pagesM.SAIDI 2023.09.13 09.58.13 DetailsLaboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- Hasim. S1Document2 pagesHasim. S1pkmsilo1No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypesetyawanankesNo ratings yet
- Instrum 301122144638 2022.11.30 14.47.40 DetailsDocument1 pageInstrum 301122144638 2022.11.30 14.47.40 DetailsLabovida RoraimaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument3 pagesTest Report: Assay Assay Version Assay TypeAlex MoralesNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument7 pagesTest Report: Assay Assay Version Assay TyperosmayaniimutzNo ratings yet
- Qa Test Format X RayDocument9 pagesQa Test Format X RayNIKHIL VERMANo ratings yet
- Wavelength accuracy test passesDocument2 pagesWavelength accuracy test passescarlos germanNo ratings yet
- PCRDocument6 pagesPCRJose F. Ramirez MendozaNo ratings yet
- P L Sharma District Hospital Meerut SHF 835Document5 pagesP L Sharma District Hospital Meerut SHF 835rajeshk.praja1997No ratings yet
- OM ClotQuant 4Document28 pagesOM ClotQuant 4Jose PersiaNo ratings yet
- Glifosato Nppe-19573Document2 pagesGlifosato Nppe-19573AS AdivNo ratings yet
- Gpti Qicl J931 Mut 019Document3 pagesGpti Qicl J931 Mut 019uselessinstaid52No ratings yet
- FCC Part 15B ICES-003, ISSUE 6, JANUARY 2016 Test Report: SZ Dji Technology Co., LTDDocument33 pagesFCC Part 15B ICES-003, ISSUE 6, JANUARY 2016 Test Report: SZ Dji Technology Co., LTDRohitNo ratings yet
- 0001ba008448 1 F1Document1 page0001ba008448 1 F1Ankush SainiNo ratings yet
- Non Destructive Testing Procedure UT, RT, MT, PT (ASME)Document63 pagesNon Destructive Testing Procedure UT, RT, MT, PT (ASME)Irvan Maruli100% (1)
- Alba Report - CompressedDocument30 pagesAlba Report - Compressedwinston11No ratings yet
- D-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703: ThyrocareDocument3 pagesD-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703: ThyrocareAryanNo ratings yet
- Car Charger CE-EMC ReportDocument21 pagesCar Charger CE-EMC Reportgqg9sw6kbgNo ratings yet
- FCC Test Report: BTL IncDocument70 pagesFCC Test Report: BTL IncLizardo Francisco Da SilvaNo ratings yet
- Certificate of Relief Valve Capacity (PROCA) 7 PDFDocument1 pageCertificate of Relief Valve Capacity (PROCA) 7 PDFAdeoye OkunoyeNo ratings yet
- FT-IR Identification ProcedureDocument6 pagesFT-IR Identification ProcedureToe PaingNo ratings yet
- Ultrasonic Exam Report Steam Drum JointsDocument3 pagesUltrasonic Exam Report Steam Drum JointsBalkishan DyavanapellyNo ratings yet
- NM002C23421596794477 - RLS 3 Converted 2Document3 pagesNM002C23421596794477 - RLS 3 Converted 2pravins93No ratings yet
- Project Owner Contractor Inspection Surveillance Report (Isr)Document20 pagesProject Owner Contractor Inspection Surveillance Report (Isr)destri_742053763No ratings yet
- Gpti Qicl J931 Mut 004Document3 pagesGpti Qicl J931 Mut 004uselessinstaid52No ratings yet
- Client: Pt. STBC Location: Autoclave 3 & 4 Report No.: 001-PAUT/RBT-STBC/XI/2022Document60 pagesClient: Pt. STBC Location: Autoclave 3 & 4 Report No.: 001-PAUT/RBT-STBC/XI/2022Rizal HidayatullahNo ratings yet
- Ultrasonic Test Procedure1Document8 pagesUltrasonic Test Procedure1MHDNo ratings yet
- Gpti Qicl J931 Mut 041Document3 pagesGpti Qicl J931 Mut 041uselessinstaid52No ratings yet
- Ultrasonic Tube to Tube Sheet ExamDocument6 pagesUltrasonic Tube to Tube Sheet ExamChandrasekhar mishraNo ratings yet
- OVP - OQ Test Protocol: Overall Test Result PASSEDDocument8 pagesOVP - OQ Test Protocol: Overall Test Result PASSEDAli RizviNo ratings yet
- Test Glo-Qc-Tm-0744Document6 pagesTest Glo-Qc-Tm-0744rx bafnaNo ratings yet
- Gpti Qicl J931 Mut 002Document2 pagesGpti Qicl J931 Mut 002uselessinstaid52No ratings yet
- GDA-GDR_TUV sismico REV00Document46 pagesGDA-GDR_TUV sismico REV00Al-KaiserNo ratings yet
- NDT Test Report NTP PDFDocument6 pagesNDT Test Report NTP PDFwawan kusnandar100% (1)
- 11-03-2020 C1 Gland Steam Super Heater - UTDocument4 pages11-03-2020 C1 Gland Steam Super Heater - UTDave CheungNo ratings yet