Professional Documents
Culture Documents
Test Report: Assay Assay Version Assay Type
Uploaded by
Melania Dessy Savitri SunarjadiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Test Report: Assay Assay Version Assay Type
Uploaded by
Melania Dessy Savitri SunarjadiCopyright:
Available Formats
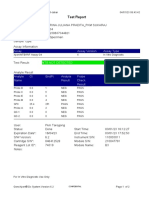
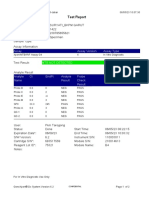
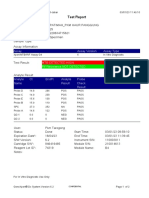
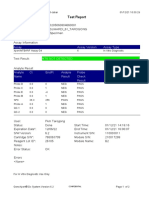
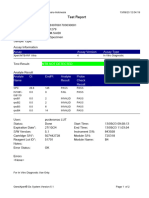
GeneXpert PC 08/28/19 12:34:22
Test Report
Patient ID: SUWARNI
Sample ID: 158
Test Type: Specimen
Sample Type:
Assay Information
Assay Assay Version Assay Type
Xpert MTB-RIF Assay G4 5 In Vitro Diagnostic
Test Result: MTB DETECTED VERY LOW;
Rif Resistance DETECTED
-
Analyte Result
Analyte Ct EndPt Analyte Probe
Name Result Check
Result
Probe D 37.9 31 NEG PASS
Probe C 31.2 115 POS PASS
Probe E 37.5 35 NEG PASS
Probe B 33.3 40 POS PASS
SPC 27.0 270 NA PASS
Probe A 33.2 83 POS PASS
QC-1 0.0 0 NEG PASS
QC-2 0.0 0 NEG PASS
User: heldajayanti
Status: Done Start Time: 08/28/19 10:33:32
Expiration Date*: 10/25/20 End Time: 08/28/19 12:15:31
S/W Version: 4.7b Instrument S/N: 811480
Cartridge S/N*: 864216068 Module S/N: 680863
Reagent Lot ID*: 55414 Module Name: A3
Notes:
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 4.7b Page 1 of 2
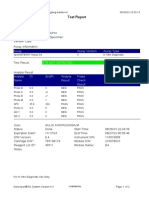
GeneXpert PC 08/28/19 12:34:22
Test Report
Error Status: OK
-
Errors
<None>
Tech. Initial/Date Supervisor Initial/Date
* indicates that a particular field is entered using a barcode scanner
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 4.7b Page 2 of 2
You might also like
- 3Document6 pages3Jose F. Ramirez MendozaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument6 pagesTest Report: Assay Assay Version Assay TypeJose F. Ramirez MendozaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay Typeyossy aprillyaNo ratings yet
- PKM BetoambariDocument6 pagesPKM Betoambarinur liylaNo ratings yet
- Ananda FebriantiDocument2 pagesAnanda FebriantiInternis RsuyarsiNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypesetyawanankesNo ratings yet
- Cihideung TCM DetailsDocument10 pagesCihideung TCM Detailsmulyadi diningrumNo ratings yet
- 2023.01.03 16.12.27 DetailsDocument2 pages2023.01.03 16.12.27 Detailsakreditasi tarogong 2023No ratings yet
- Nurbaya 2023.02.02 14.49.06 DetailsDocument2 pagesNurbaya 2023.02.02 14.49.06 DetailsRahmatul LailiNo ratings yet
- 2023.05.06 08.22.15 DetailsDocument2 pages2023.05.06 08.22.15 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 15.25.33 DetailsDocument2 pages2023.01.03 15.25.33 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 08.06.58 DetailsDocument2 pages2023.01.03 08.06.58 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 11.48.53 DetailsDocument2 pages2023.01.03 11.48.53 Detailsakreditasi tarogong 2023No ratings yet
- SUKARMINI-DPM DR ABDUL GHOFIRDocument2 pagesSUKARMINI-DPM DR ABDUL GHOFIRlaboratorium mitra utamaNo ratings yet
- Bungga Hobrouw (R.anak)Document2 pagesBungga Hobrouw (R.anak)lukas mansnandifuNo ratings yet
- APIHDocument2 pagesAPIHpuskesmas cigeulisNo ratings yet
- IWA 29TH PKM TRG 2021.12.01 08.35.17 DetailsDocument4 pagesIWA 29TH PKM TRG 2021.12.01 08.35.17 Detailsakreditasi tarogong 2023No ratings yet
- Hasim. S1Document2 pagesHasim. S1pkmsilo1No ratings yet
- NANI K 46TH PASUNDAN 2021.12.01 08.36.02 DetailsDocument4 pagesNANI K 46TH PASUNDAN 2021.12.01 08.36.02 Detailsakreditasi tarogong 2023No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument3 pagesTest Report: Assay Assay Version Assay TypeAlex MoralesNo ratings yet
- 2023.01.03 09.59.10 DetailsDocument2 pages2023.01.03 09.59.10 Detailsakreditasi tarogong 2023No ratings yet
- SUHARDI 61 TAROGONG 2021.12.01 14.18.16 DetailsDocument2 pagesSUHARDI 61 TAROGONG 2021.12.01 14.18.16 Detailsakreditasi tarogong 2023No ratings yet
- Tamron IDocument2 pagesTamron IEri OiNo ratings yet
- Rsi Sa 12-10-2023Document6 pagesRsi Sa 12-10-2023Laboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- M.SAIDI 2023.09.13 09.58.13 DetailsDocument2 pagesM.SAIDI 2023.09.13 09.58.13 DetailsLaboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- Tool Inspection Log For Customers 01 10 19 With 3 InchDocument1 pageTool Inspection Log For Customers 01 10 19 With 3 InchcodyNo ratings yet
- LPL - Lpl-Rohini (National Reference Lab) Sector - 18, Block - E Rohini DELHI 110085Document2 pagesLPL - Lpl-Rohini (National Reference Lab) Sector - 18, Block - E Rohini DELHI 110085Ss LaptopNo ratings yet
- Variant Ii Hemoglobin Testing System For Hba: Bio-Rad LaboratoriesDocument6 pagesVariant Ii Hemoglobin Testing System For Hba: Bio-Rad LaboratoriesBhageshwar ChaudharyNo ratings yet
- CertificateofAnalysis 2020225 521261 PDFDocument2 pagesCertificateofAnalysis 2020225 521261 PDFzarlyNo ratings yet
- RNA Clean 17-8 - Eukaryote Total RNA Nano - DE72905431 - 2022-08-18 - 11-23-22Document15 pagesRNA Clean 17-8 - Eukaryote Total RNA Nano - DE72905431 - 2022-08-18 - 11-23-22Sara SolimanNo ratings yet
- EMC Pro1040 Pro1050 2no363 PV701 Pro1050 Pro1040 N36201B N36200BDocument10 pagesEMC Pro1040 Pro1050 2no363 PV701 Pro1050 Pro1040 N36201B N36200BTayfun SezişNo ratings yet
- 23 11 21 ExtractProtocolJoan GoodLadderDocument18 pages23 11 21 ExtractProtocolJoan GoodLadderle.gonzalez2075No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument7 pagesTest Report: Assay Assay Version Assay TyperosmayaniimutzNo ratings yet
- 05 Brochure CA-660 - PDocument2 pages05 Brochure CA-660 - Plaboratorium terintegrasiNo ratings yet
- DocUReader 2 PRO Uputstvo enDocument66 pagesDocUReader 2 PRO Uputstvo enDarko MaksimovicNo ratings yet
- PCRDocument6 pagesPCRJose F. Ramirez MendozaNo ratings yet
- Mobidiag LIS Protocol (Mobidiag LIS Service 1.4.0) V4-0Document24 pagesMobidiag LIS Protocol (Mobidiag LIS Service 1.4.0) V4-0Runy RunyNo ratings yet
- Wavelength accuracy test passesDocument2 pagesWavelength accuracy test passescarlos germanNo ratings yet
- الصيانة الدورية اللتراساوندDocument2 pagesالصيانة الدورية اللتراساوندtinep0493No ratings yet
- VARGA ResultsDocument3 pagesVARGA ResultsCocos MirelNo ratings yet
- Name Received Collected Dummy Z839: InterpretationDocument2 pagesName Received Collected Dummy Z839: Interpretationcra storeNo ratings yet
- TEG® Analysis: Sample Data: Normal Values: CPT/Billing Codes: UnitsDocument1 pageTEG® Analysis: Sample Data: Normal Values: CPT/Billing Codes: UnitsDendyNo ratings yet
- Summary - 803569 - 2023 01 27 - 08 35 16Document2 pagesSummary - 803569 - 2023 01 27 - 08 35 16Satriawan SyahNo ratings yet
- DocUReader 2 Operators ManualDocument85 pagesDocUReader 2 Operators ManualKinnari BhattNo ratings yet
- LPL-ROHINI PML-RARA GENE REARRANGEMENT TEST REPORTDocument2 pagesLPL-ROHINI PML-RARA GENE REARRANGEMENT TEST REPORTsmishra1lnctNo ratings yet
- CertificateofAnalysis 2021 12 1 971796Document2 pagesCertificateofAnalysis 2021 12 1 971796adiazcalidadNo ratings yet
- Tuv Report: Sample InformationDocument1 pageTuv Report: Sample InformationBiotomy LifesciencesNo ratings yet
- Final Report NAUTO PILOT DTADocument4 pagesFinal Report NAUTO PILOT DTASmart PelicanNo ratings yet
- COVID-19 PCR Test Report for Saneer Puthiya ValappilDocument1 pageCOVID-19 PCR Test Report for Saneer Puthiya ValappilSANEER P VNo ratings yet
- OM ClotQuant 4Document28 pagesOM ClotQuant 4Jose PersiaNo ratings yet
- PF2P5MKX-2023-09-18-231029Document16 pagesPF2P5MKX-2023-09-18-231029tikagenouNo ratings yet
- Final Report UT Inspection BearingDocument5 pagesFinal Report UT Inspection BearingEko PurwantoNo ratings yet
- EMC Pro1040 Pro1050 2No363A PV701 Pro1050 40 N36201B 0B X SignedDocument10 pagesEMC Pro1040 Pro1050 2No363A PV701 Pro1050 40 N36201B 0B X SignedTayfun SezişNo ratings yet
- A-Star Testing & Inspection (S) Pte LTD: Magnetic Particle Testing ReportDocument4 pagesA-Star Testing & Inspection (S) Pte LTD: Magnetic Particle Testing ReportHari KarthickNo ratings yet
- PARACETAMOL MethodDocument3 pagesPARACETAMOL MethodRubén RuelasNo ratings yet
- EKAMJOTDocument1 pageEKAMJOTAnshi SharmaNo ratings yet
- Instruction For C-Reactive Protein (CRP) Detection Kit (Nephelometry)Document2 pagesInstruction For C-Reactive Protein (CRP) Detection Kit (Nephelometry)Muhammad KhalidNo ratings yet
- ANEXO7Document14 pagesANEXO7liuming farfanNo ratings yet
- Lab 9Document13 pagesLab 9Hassaan SaeedNo ratings yet