Professional Documents
Culture Documents
Test Report: Assay Assay Version Assay Type
Uploaded by
setyawanankes0 ratings0% found this document useful (0 votes)
18 views2 pagesOriginal Title
2020.07.18_SITI RAFIKASARIY_BRINGIN
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
18 views2 pagesTest Report: Assay Assay Version Assay Type
Uploaded by
setyawanankesCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
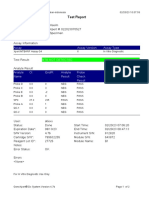
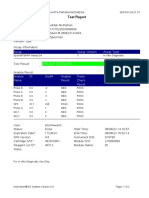
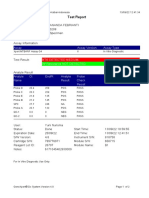
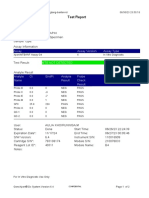
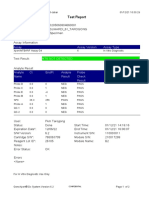
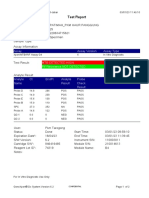
GeneXpert-810604-RSUD DR SOEROTO-NGAWI-INDONESIA 07/18/20 13:25:11
Test Report
Patient ID: 3521075111950002
Sample ID: SITI RAFIKASARIY_BRINGIN
Test Type: Specimen
Sample Type:
Assay Information
Assay Assay Version Assay Type
Xpert MTB-RIF Assay G4 5 In Vitro Diagnostic
Test Result: MTB NOT DETECTED
-
Analyte Result
Analyte Ct EndPt Analyte Probe
Name Result Check
Result
Probe D 0.0 2 NEG PASS
Probe C 0.0 17 NEG PASS
Probe E 0.0 -4 NEG PASS
Probe B 0.0 2 NEG PASS
SPC 24.0 284 PASS PASS
Probe A 0.0 9 NEG PASS
QC-1 0.0 0 NEG PASS
QC-2 0.0 0 NEG PASS
User: Lab. RSUD DR. SOEROTO
Status: Done Start Time: 07/18/20 09:57:10
Expiration Date*: 10/31/21 End Time: 07/18/20 11:38:13
S/W Version: 4.7b Instrument S/N: 810604
Cartridge S/N*: 724725804 Module S/N: 601426
Reagent Lot ID*: 25503 Module Name: B4
Notes:
Error Status: OK
-
Errors
<None>
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 4.7b Page 1 of 2
GeneXpert-810604-RSUD DR SOEROTO-NGAWI-INDONESIA 07/18/20 13:25:11
Test Report
Tech. Initial/Date Supervisor Initial/Date
* indicates that a particular field is entered using a barcode scanner
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 4.7b Page 2 of 2
You might also like
- Eti Max3000Document3 pagesEti Max3000Ana AsmaraNo ratings yet
- Bidh Lab: Covid-19 Test Authorized by National Public Health Laboratory (NPHL), Nepal Microbiology ReportDocument1 pageBidh Lab: Covid-19 Test Authorized by National Public Health Laboratory (NPHL), Nepal Microbiology Reportrayguntan100% (1)
- Patients Profile: Not Detected NegativeDocument2 pagesPatients Profile: Not Detected NegativeELLIE JAMES PLACIONo ratings yet
- Brochure PDFDocument72 pagesBrochure PDFkisa guyNo ratings yet
- 3Document6 pages3Jose F. Ramirez MendozaNo ratings yet
- Amur Mohammed Amur Al BassamiDocument1 pageAmur Mohammed Amur Al BassamiAamer AlBassamiNo ratings yet
- SR - No Investigation Observed Value Reference Range: InterpretationDocument1 pageSR - No Investigation Observed Value Reference Range: InterpretationVinod KumarNo ratings yet
- 03-12-2021 7:59 Am Covid-Sudharma Lab Wandoor: Molecular Biology ReportDocument2 pages03-12-2021 7:59 Am Covid-Sudharma Lab Wandoor: Molecular Biology ReportRinu jasNo ratings yet
- Abstract:: Keywords: COVID-19, NAAT, Reagents, Low-CostDocument25 pagesAbstract:: Keywords: COVID-19, NAAT, Reagents, Low-Costप्रशान्त वाईबाNo ratings yet
- Pointcare Now Brochure EngDocument10 pagesPointcare Now Brochure EngAnnisa ChaeraniNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument3 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodPraveen KumarNo ratings yet
- DR Poli Obs Ii Mug 01.03.19Document22 pagesDR Poli Obs Ii Mug 01.03.19mughanNo ratings yet
- Product Presentation Nova Blood Gas AnalyzerDocument38 pagesProduct Presentation Nova Blood Gas Analyzerlaboratorium rsdmadani100% (1)
- ST2134 Trainer Kit ManualDocument171 pagesST2134 Trainer Kit ManualJeevan Prakash100% (1)
- Webinar 022912 Sce Enumeration Kit PDFDocument112 pagesWebinar 022912 Sce Enumeration Kit PDFRafika Rara100% (1)
- Navios Flow Cytometer: Optimize Workflow With A Cytometer That Brings To Your LabDocument6 pagesNavios Flow Cytometer: Optimize Workflow With A Cytometer That Brings To Your LabcandiddreamsNo ratings yet
- Soil Moisture Sensor PDFDocument5 pagesSoil Moisture Sensor PDFM. Yusup100% (1)
- Ventilator Mindray Syno Vent E3Document2 pagesVentilator Mindray Syno Vent E3erika100% (1)
- Lijo John Kennedy - F - 21012021213850Document1 pageLijo John Kennedy - F - 21012021213850Lijo John100% (1)
- VIDAS miniVIDAS ServiceManual 31-01-2011 (1) - 2Document21 pagesVIDAS miniVIDAS ServiceManual 31-01-2011 (1) - 2metana90No ratings yet
- Manual ST 100 BDocument101 pagesManual ST 100 BSmart BiomedicalNo ratings yet
- MODEM Schematic Baseband SchematicDocument55 pagesMODEM Schematic Baseband SchematicCesar Albarran100% (1)
- Boeco Autoclave Bte-23D: FeaturesDocument1 pageBoeco Autoclave Bte-23D: FeaturesAdrian Gomez BaldeonNo ratings yet
- CBA MouseTh1Th2 Kit ManualDocument52 pagesCBA MouseTh1Th2 Kit ManualendopetNo ratings yet
- Zainab Kadhim Saeed AlmusawiDocument1 pageZainab Kadhim Saeed AlmusawiAliya AbdulazizNo ratings yet
- Form. Admin LabDocument5 pagesForm. Admin LabDadang MardiawanNo ratings yet
- DCHS-C05 850 05 02 02Document50 pagesDCHS-C05 850 05 02 02ОлександрNo ratings yet
- Spesifikasi Indiko PLUS PDFDocument1 pageSpesifikasi Indiko PLUS PDFpigWaterNo ratings yet
- RQ-BCR-ABL p210 One-Step Manual E20170119Document22 pagesRQ-BCR-ABL p210 One-Step Manual E20170119moutasim mohammadNo ratings yet
- Pathology 09.11.2020 11.09.01.034Document1 pagePathology 09.11.2020 11.09.01.034Subhajit RoyNo ratings yet
- Service Manual - Selectra Pro S - 6003-500-450-01Document344 pagesService Manual - Selectra Pro S - 6003-500-450-01joseavilaNo ratings yet
- Kesehatan Daerah Militer Vi/Mulawarman: Jl. Tanjungpura VI Telp. (0542) 8502682 Balikpapan - Kalimantan TimurDocument1 pageKesehatan Daerah Militer Vi/Mulawarman: Jl. Tanjungpura VI Telp. (0542) 8502682 Balikpapan - Kalimantan TimurNikenNo ratings yet
- Biot Yg I Biotime Fluorescence Immunoassay AnalyzerDocument2 pagesBiot Yg I Biotime Fluorescence Immunoassay Analyzerxuseen maxamedNo ratings yet
- MicroScan BrochureDocument6 pagesMicroScan BrochureShifaNo ratings yet
- P25 Firmware Update Guide v1.1Document13 pagesP25 Firmware Update Guide v1.1Francisco José PapiNo ratings yet
- Orphee Mythic 22-CT Hematology Analyzer - User ManualDocument102 pagesOrphee Mythic 22-CT Hematology Analyzer - User ManualAna Laura Ochoa ZepedaNo ratings yet
- Hasim. S1Document2 pagesHasim. S1pkmsilo1No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay Typeyossy aprillyaNo ratings yet
- PKM BetoambariDocument6 pagesPKM Betoambarinur liylaNo ratings yet
- Ananda FebriantiDocument2 pagesAnanda FebriantiInternis RsuyarsiNo ratings yet
- Nurbaya 2023.02.02 14.49.06 DetailsDocument2 pagesNurbaya 2023.02.02 14.49.06 DetailsRahmatul LailiNo ratings yet
- Cihideung TCM DetailsDocument10 pagesCihideung TCM Detailsmulyadi diningrumNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypeMelania Dessy Savitri SunarjadiNo ratings yet
- APIHDocument2 pagesAPIHpuskesmas cigeulisNo ratings yet
- IWA 29TH PKM TRG 2021.12.01 08.35.17 DetailsDocument4 pagesIWA 29TH PKM TRG 2021.12.01 08.35.17 Detailsakreditasi tarogong 2023No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument6 pagesTest Report: Assay Assay Version Assay TypeJose F. Ramirez MendozaNo ratings yet
- 2023.05.06 08.22.15 DetailsDocument2 pages2023.05.06 08.22.15 Detailsakreditasi tarogong 2023No ratings yet
- NANI K 46TH PASUNDAN 2021.12.01 08.36.02 DetailsDocument4 pagesNANI K 46TH PASUNDAN 2021.12.01 08.36.02 Detailsakreditasi tarogong 2023No ratings yet
- Tamron IDocument2 pagesTamron IEri OiNo ratings yet
- Rsi Sa 12-10-2023Document6 pagesRsi Sa 12-10-2023Laboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- Bungga Hobrouw (R.anak)Document2 pagesBungga Hobrouw (R.anak)lukas mansnandifuNo ratings yet
- M.SAIDI 2023.09.13 09.58.13 DetailsDocument2 pagesM.SAIDI 2023.09.13 09.58.13 DetailsLaboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- 2023.01.03 08.06.58 DetailsDocument2 pages2023.01.03 08.06.58 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 16.12.27 DetailsDocument2 pages2023.01.03 16.12.27 Detailsakreditasi tarogong 2023No ratings yet
- SUHARDI 61 TAROGONG 2021.12.01 14.18.16 DetailsDocument2 pagesSUHARDI 61 TAROGONG 2021.12.01 14.18.16 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 15.25.33 DetailsDocument2 pages2023.01.03 15.25.33 Detailsakreditasi tarogong 2023No ratings yet
- SUKARMINI-DPM DR ABDUL GHOFIRDocument2 pagesSUKARMINI-DPM DR ABDUL GHOFIRlaboratorium mitra utamaNo ratings yet
- 2023.01.03 11.48.53 DetailsDocument2 pages2023.01.03 11.48.53 Detailsakreditasi tarogong 2023No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument3 pagesTest Report: Assay Assay Version Assay TypeAlex MoralesNo ratings yet
- 2023.01.03 09.59.10 DetailsDocument2 pages2023.01.03 09.59.10 Detailsakreditasi tarogong 2023No ratings yet