Professional Documents
Culture Documents
2023.01.03 11.48.53 Details
Uploaded by
akreditasi tarogong 20230 ratings0% found this document useful (0 votes)
11 views2 pagesOriginal Title
230635800121_2023.01.03_11.48.53_details
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
11 views2 pages2023.01.03 11.48.53 Details
Uploaded by
akreditasi tarogong 2023Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
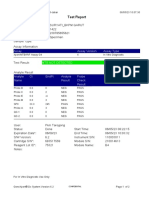
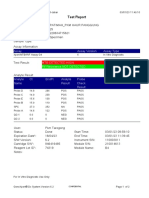
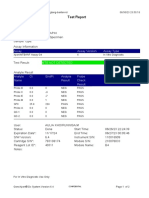
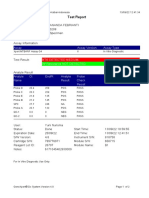
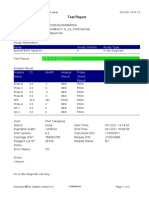
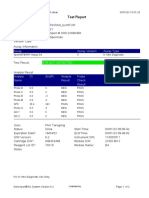
110003011-PKM Tarogong-Garut-Jabar 03/01/23 13:49:08
Test Report
Patient ID: AAN HAYATI_PKM TAROGONG
Patient ID 2: 30
Sample ID: 230635800121
Test Type: Specimen
Sample Type:
Assay Information
Assay Assay Version Assay Type
Xpert MTB-RIF Assay G4 6 In Vitro Diagnostic
Test Result: MTB NOT DETECTED
-
Analyte Result
Analyte Ct EndPt Analyte Probe
Name Result Check
Result
Probe D 0.0 1 NEG PASS
Probe C 0.0 4 NEG PASS
Probe E 0.0 0 NEG PASS
Probe B 0.0 4 NEG PASS
SPC 24.2 263 PASS PASS
Probe A 0.0 2 NEG PASS
QC-1 0.0 0 NEG PASS
QC-2 0.0 0 NEG PASS
User: Pkm Tarogong
Status: Done Start Time: 03/01/23 11:48:53
Expiration Date*: 16/04/23 End Time: 03/01/23 13:29:26
S/W Version: 6.2 Instrument S/N: 110003011
Cartridge S/N*: 846412529 Module S/N: 210047791
Reagent Lot ID*: 74319 Module Name: B3
Notes:
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 6.2 CONFIDENTIAL Page 1 of 2
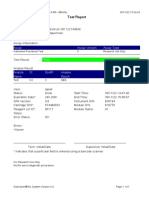
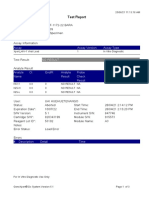
110003011-PKM Tarogong-Garut-Jabar 03/01/23 13:49:08
Test Report
Error Status: OK
-
Errors
<None>
Tech. Initial/Date Supervisor Initial/Date
* indicates that a particular field is entered using a barcode scanner
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 6.2 CONFIDENTIAL Page 2 of 2
You might also like
- 2023.01.03 16.12.27 DetailsDocument2 pages2023.01.03 16.12.27 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 08.06.58 DetailsDocument2 pages2023.01.03 08.06.58 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 15.25.33 DetailsDocument2 pages2023.01.03 15.25.33 Detailsakreditasi tarogong 2023No ratings yet
- 2023.05.06 08.22.15 DetailsDocument2 pages2023.05.06 08.22.15 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 09.59.10 DetailsDocument2 pages2023.01.03 09.59.10 Detailsakreditasi tarogong 2023No ratings yet
- NANI K 46TH PASUNDAN 2021.12.01 08.36.02 DetailsDocument4 pagesNANI K 46TH PASUNDAN 2021.12.01 08.36.02 Detailsakreditasi tarogong 2023No ratings yet
- IWA 29TH PKM TRG 2021.12.01 08.35.17 DetailsDocument4 pagesIWA 29TH PKM TRG 2021.12.01 08.35.17 Detailsakreditasi tarogong 2023No ratings yet
- SUHARDI 61 TAROGONG 2021.12.01 14.18.16 DetailsDocument2 pagesSUHARDI 61 TAROGONG 2021.12.01 14.18.16 Detailsakreditasi tarogong 2023No ratings yet
- SUKARMINI-DPM DR ABDUL GHOFIRDocument2 pagesSUKARMINI-DPM DR ABDUL GHOFIRlaboratorium mitra utamaNo ratings yet
- APIHDocument2 pagesAPIHpuskesmas cigeulisNo ratings yet
- Cihideung TCM DetailsDocument10 pagesCihideung TCM Detailsmulyadi diningrumNo ratings yet
- PKM BetoambariDocument6 pagesPKM Betoambarinur liylaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay Typeyossy aprillyaNo ratings yet
- 3Document6 pages3Jose F. Ramirez MendozaNo ratings yet
- Tamron IDocument2 pagesTamron IEri OiNo ratings yet
- Nurbaya 2023.02.02 14.49.06 DetailsDocument2 pagesNurbaya 2023.02.02 14.49.06 DetailsRahmatul LailiNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypeMelania Dessy Savitri SunarjadiNo ratings yet
- Bungga Hobrouw (R.anak)Document2 pagesBungga Hobrouw (R.anak)lukas mansnandifuNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument6 pagesTest Report: Assay Assay Version Assay TypeJose F. Ramirez MendozaNo ratings yet
- Hasim. S1Document2 pagesHasim. S1pkmsilo1No ratings yet
- M.SAIDI 2023.09.13 09.58.13 DetailsDocument2 pagesM.SAIDI 2023.09.13 09.58.13 DetailsLaboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- Rsi Sa 12-10-2023Document6 pagesRsi Sa 12-10-2023Laboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- Instrum 301122144638 2022.11.30 14.47.40 DetailsDocument1 pageInstrum 301122144638 2022.11.30 14.47.40 DetailsLabovida RoraimaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypesetyawanankesNo ratings yet
- Ananda FebriantiDocument2 pagesAnanda FebriantiInternis RsuyarsiNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument3 pagesTest Report: Assay Assay Version Assay TypeAlex MoralesNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument7 pagesTest Report: Assay Assay Version Assay TyperosmayaniimutzNo ratings yet
- Wavelength accuracy test passesDocument2 pagesWavelength accuracy test passescarlos germanNo ratings yet
- 0001ba008448 1 F1Document1 page0001ba008448 1 F1Ankush SainiNo ratings yet
- CNT230022-5 C3 HRSG LP Drum - MTreportDocument6 pagesCNT230022-5 C3 HRSG LP Drum - MTreportDave CheungNo ratings yet
- Qa Test Format X RayDocument9 pagesQa Test Format X RayNIKHIL VERMANo ratings yet
- OVP - OQ Test Protocol: Overall Test Result PASSEDDocument8 pagesOVP - OQ Test Protocol: Overall Test Result PASSEDAli RizviNo ratings yet
- PCRDocument6 pagesPCRJose F. Ramirez MendozaNo ratings yet
- 31-03-2020 C5 HRSG - LP Reedwater To HRSG DRUM Level Control Valve - PTDocument3 pages31-03-2020 C5 HRSG - LP Reedwater To HRSG DRUM Level Control Valve - PTDave CheungNo ratings yet
- Meghna PVC LTD (Pet Division) QAC Department Shift Chemist Log BookDocument5 pagesMeghna PVC LTD (Pet Division) QAC Department Shift Chemist Log Bookswapon kumar shillNo ratings yet
- VARGA ResultsDocument3 pagesVARGA ResultsCocos MirelNo ratings yet
- Variant Ii Turbo Hemoglobin Testing System: Touseasanaidin The Diagnosis of DiabetesDocument6 pagesVariant Ii Turbo Hemoglobin Testing System: Touseasanaidin The Diagnosis of DiabetesKetevan MigriauliNo ratings yet
- Sars-Cov2 (Covid-19) Real Time RT PCR TestDocument2 pagesSars-Cov2 (Covid-19) Real Time RT PCR TestGEO MERINNo ratings yet
- A-Star Testing & Inspection (S) Pte LTD: Magnetic Particle Testing ReportDocument4 pagesA-Star Testing & Inspection (S) Pte LTD: Magnetic Particle Testing ReportHari KarthickNo ratings yet
- MIghty Machine RejectDocument1 pageMIghty Machine RejectMohammad Shanawaz MNo ratings yet
- CNT200020-3 C3 HRSG HP Drum - MT ReportDocument6 pagesCNT200020-3 C3 HRSG HP Drum - MT ReportDave CheungNo ratings yet
- 11-03-2020 C1 Gland Steam Super Heater - UTDocument4 pages11-03-2020 C1 Gland Steam Super Heater - UTDave CheungNo ratings yet
- NDT Test Report NTP PDFDocument6 pagesNDT Test Report NTP PDFwawan kusnandar100% (1)
- Autotune-SourceTune High Matrix 1350-20231201-125812004Document6 pagesAutotune-SourceTune High Matrix 1350-20231201-125812004luis avitNo ratings yet
- Worksheet - PTDocument5 pagesWorksheet - PTDave CheungNo ratings yet
- Certificado Test ReportDocument28 pagesCertificado Test ReportdronebessaNo ratings yet
- Berhad I Endurance Anchorage: Non-Destructive TestDocument2 pagesBerhad I Endurance Anchorage: Non-Destructive TestNua JamilNo ratings yet
- CertificateofAnalysis 2021 12 1 971796Document2 pagesCertificateofAnalysis 2021 12 1 971796adiazcalidadNo ratings yet
- Variant Ii Hemoglobin Testing System For Hba: Bio-Rad LaboratoriesDocument6 pagesVariant Ii Hemoglobin Testing System For Hba: Bio-Rad LaboratoriesBhageshwar ChaudharyNo ratings yet
- LabUPlus SerialconnectDocument7 pagesLabUPlus SerialconnectJose Perez PerezNo ratings yet
- DocUReader 2 PRO Uputstvo enDocument66 pagesDocUReader 2 PRO Uputstvo enDarko MaksimovicNo ratings yet
- 24-02-2020 C1 - Turbine Bucket Rockwire Tab - PTDocument3 pages24-02-2020 C1 - Turbine Bucket Rockwire Tab - PTDave CheungNo ratings yet
- Calibration ReportDocument119 pagesCalibration ReportFadilah Fauzan NugrahaNo ratings yet
- Bureau of Indian Standards BIS, Patna Branch Laboratory (PBL)Document5 pagesBureau of Indian Standards BIS, Patna Branch Laboratory (PBL)sunil kumarNo ratings yet
- DocUReader 2 Operators ManualDocument85 pagesDocUReader 2 Operators ManualKinnari BhattNo ratings yet
- WHO SARS-CoV-2 PCR Proficiency Testing ResultsDocument2 pagesWHO SARS-CoV-2 PCR Proficiency Testing ResultspermencokelatNo ratings yet
- Alba Report - CompressedDocument30 pagesAlba Report - Compressedwinston11No ratings yet
- Report of F - CH Maribel Snigitha CiceroDocument2 pagesReport of F - CH Maribel Snigitha CiceroR.Pearlsis SophiNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- Xpert M 030123080450 2023.01.03 08.05.42 DetailsDocument2 pagesXpert M 030123080450 2023.01.03 08.05.42 Detailsakreditasi tarogong 2023No ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet