Professional Documents
Culture Documents
Analysis of Phosphate in Fertilizer and Drink
Uploaded by
Elen MariOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Analysis of Phosphate in Fertilizer and Drink
Uploaded by
Elen MariCopyright:
Available Formats
Nana Mirotadze
Lab partner: Elene Ivaniashvili
G3
30/05/2019
Chem 201, Experiment 2
Analysis of Phosphate
Procedure: I followed procedure of Chem 201 lab manual. Prelab pages 3-4.
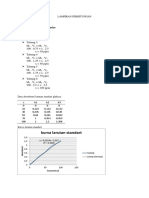
Experimental data:
Part 1 calibration
Table 1, Phosphate+ammonium vanadomolybdate
Wavelength (nm) absorbance
375 1.33
385 0.99
395 0.77
405 0.60
415 0.48
Absorbance vs Wavelength
1.4
1.2
1
absorbance
0.8
0.6
0.4
0.2
0
370 375 380 385 390 395 400 405 410 415 420
wavelength (nm)
The highest absorbance was at 375 nm it was 1.33 (AT)
Table 2, ammonium vanadomolybdate
Absorbance at 375 nm (AR) 0.16
Concentration of stock solution (M) 2.5x10^-4
Part 2
Fertilizer 100g/L
At first, we prepared 1:30 solution but absorbance was more than 1.7 it was 2.54, next we prepared 1:40
solution it was 2.50 so next we prepared 1:80 solution absorbance was 1.75 and finally we prepared 1:160
solution by adding 4mL water to 3rd 4mL solution and absorbance was 0.93.
Table 3
concentration Absorbance
1:30 2.54
1:40 2.50
1:80 1.75
1:160 0.93
Absorbance of 1:160 solution table 4
Trial 1 0.93
Trial 2 0.92
Trial 3 0.93
Part 3
Energy drink Gatorade Frost blue drink solution 1:30 absorbance Table 4
Trial 1 0.64
Trial 2 0.63
Trial 3 0.63
Calculated results:
Part 1 calibration
Absorbance of phosphate complex AP 1.17
𝜀𝑙 of phosphate complex M-1 4.76x10^3
Part 2 fertilizer
Trial 1 Trial 2 Trial 2
AP 0.77 0.76 0.77
Concentration of 1.6x10^-4 1.58x10^-4 1.6x10^-4
diluted solution (1:160)
(M)
Concentration of 0.026 0.0252 0.026
original solution (M)
g/L of P2O5 1.8 1.79 1.8
% P2O5 1.8 1.79 1.8
average 1.8
Standard deviation ±7x10^-3
Part 3 Gatorade frost 946mL
Trial 1 Trial 2 Trial 3
AP 0.48 0.47 0.47
Concentration of 1.0x10^-4 9.8x10^-5 9.8x10^-5
diluted solution (1:30)
(M)
Concentration of 3.0x10^-3 2.9x10^-3 2.9x10^-3
original solution (M)
Moles of PO43- in the 2.8x10^-3 2.7x10^-3 2.7x10^-3
bottle
Mass of PO43- (mg) 2.6x10^2 2.56x10^2 2.56x10^2
Average mass of PO43- 2.6x10^2
(mg)
Standard deviation ±4
Sample calculations
Part 1 calibration
𝐴𝑝 = 𝐴 𝑇 − 𝐴𝑅 = 1.33 − 0.16 = 1.17
𝐴𝑃 1.17
𝜀𝑙 = = = 4.8 × 103 𝑀−1
𝑐 2.5 × 10−4 𝑀
Part 2 fertilizer
𝐴𝑃 = 0.93 − 0.16 = 0.77
𝐴𝑃 0.77
𝑐(𝑑𝑖𝑙𝑢𝑡𝑒𝑑) = = = 1.6042 × 10−4 𝑀
𝜀𝑙 4.8 × 103 𝑀−1
𝑐(2) × 𝑉(2) 1.6 × 10−4 𝑀 × 160𝑚𝑙
𝑐1 (𝑜𝑟𝑖𝑔𝑖𝑛𝑎𝑙) = = = 0.026𝑀
𝑉(1) 1𝑚𝑙
𝑔 1𝑚𝑜𝑙 P2 O5
𝑜𝑓 P2 O5 = [PO4 3− ] × 𝑚𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 𝑜𝑓 P2 O5 ×
𝐿 2 𝑚𝑜𝑙 PO4 3−
141.9𝑔 1 1.8𝑔
= 0.026𝑀 × × =
𝑚𝑜𝑙 2 𝐿
𝑔
𝑜𝑓 P2 O5 1.8𝑔/𝐿
% P2 O5 = 100 × 𝑔 𝐿 = 100 × = 1.8%
𝑜𝑓 𝑓𝑒𝑟𝑡𝑖𝑙𝑖𝑧𝑒𝑟 100𝑔/𝐿
𝐿
1.8% + 1.79% + 1.8%
𝐴𝑣𝑒𝑟𝑎𝑔𝑒(%𝑃2𝑂5) = = 1.8%
3
(1.8 − 1.8)2 + (1.8 − 1.79)2 + (1.8 − 1.8)2
𝑠𝑡𝑎𝑛𝑑𝑎𝑟𝑑 𝑑𝑒𝑣𝑖𝑎𝑡𝑖𝑜𝑛 = √ = 7 × 10−3
2
Part 3 Gatorade Frost blue drink
𝐴𝑃 = 0.64 − 0.16 = 0.48
𝐴(𝑝) 0.48
𝑐(𝑑𝑖𝑙𝑢𝑡𝑒𝑑 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛) = = = 1.0 × 10−4 𝑀
𝜀𝑙 4.8 × 103 𝑀−1
1.00 × 10−4 𝑀 × 30𝑚𝑙
𝑐(𝑜𝑟𝑖𝑔𝑖𝑛𝑎𝑙 𝑠𝑙𝑢𝑡𝑖𝑜𝑛) = = 3.0 × 10−3 𝑀
1𝑚𝑙
𝑛(𝑃𝑂43− ) = 0.946𝐿 × 3.0 × 10−3 𝑀 = 2.8 × 10−3 𝑚𝑜𝑙
94.97𝑔
𝑚(𝑃𝑂43− ) = 2.8 × 10−3 𝑚𝑜𝑙 × = 0.26𝑔
𝑚𝑜𝑙
1000𝑚𝑔
0.26𝑔 × = 2.6 × 102 𝑚𝑔
1𝑔
Average mass=2.6x10^2mg
(260 − 260)2 + (260 − 256)2 + (260 − 256)2
𝑠𝑡𝑎𝑛𝑑𝑎𝑟𝑑 𝑑𝑒𝑣𝑖𝑎𝑡𝑖𝑜𝑛 = √ =4
2
Discussion
You might also like
- Compound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesFrom EverandCompound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesNo ratings yet
- Lab Report Iodine Clock ReactionDocument6 pagesLab Report Iodine Clock ReactionYoonseo (Elin) ChaNo ratings yet
- DistillationDocument8 pagesDistillationfarahalsayed64No ratings yet
- Beer Lambert Law Problems Detailed Model Answer PDFDocument2 pagesBeer Lambert Law Problems Detailed Model Answer PDFMahmoud Elshahawy83% (6)
- Mass Transfer ReportDocument89 pagesMass Transfer ReportPRALAY GEDAMNo ratings yet
- #15 Lab ReportDocument6 pages#15 Lab ReportAli Ib TarshaNo ratings yet
- HNNKNDocument4 pagesHNNKNNadia UmmahNo ratings yet
- Chapter 3 - Distillation Column Design PDFDocument54 pagesChapter 3 - Distillation Column Design PDFSyukri Zainuddin100% (6)
- Want Chemistry Games, Drills, Tests and More? You Need To Become An !Document18 pagesWant Chemistry Games, Drills, Tests and More? You Need To Become An !Liezl ValienteNo ratings yet
- Full Report On: Janelle Allyza Conti Earlene Lagasca Jose Lorenzo ManansalaDocument6 pagesFull Report On: Janelle Allyza Conti Earlene Lagasca Jose Lorenzo ManansalaTricia CentenoNo ratings yet
- Exp 6 N&MDocument9 pagesExp 6 N&MvlcjNo ratings yet
- Lab Report-13: Environmental Chemistry (ENE-213) Course Instructor: Dr. Sofia BaigDocument7 pagesLab Report-13: Environmental Chemistry (ENE-213) Course Instructor: Dr. Sofia BaigHaniya SiddiqueNo ratings yet
- Abbie Carr Lab 5Document6 pagesAbbie Carr Lab 5abbie carrNo ratings yet
- Filtration Resistance ComparisonDocument11 pagesFiltration Resistance ComparisonAnnisha Izzatie100% (1)
- Spectrophotometric Determination of pKaDocument37 pagesSpectrophotometric Determination of pKaNikko ManaleseNo ratings yet
- Determination of PolyphenolDocument17 pagesDetermination of PolyphenolOnline NinaNo ratings yet
- Lab 3 (Determination of Fatty Acids) PDFDocument17 pagesLab 3 (Determination of Fatty Acids) PDFnur syaza haniNo ratings yet
- LIQUID-LIQUID EXTRACTION EFFECT OF TIMEDocument21 pagesLIQUID-LIQUID EXTRACTION EFFECT OF TIMEJimit PNo ratings yet
- Determination of Ideality of Solutions LabDocument10 pagesDetermination of Ideality of Solutions LabLuluaNo ratings yet
- Data Sheet FR 5Document4 pagesData Sheet FR 5Claire GreciaNo ratings yet
- Chem 201 Experiment 2 - Lab ReportDocument3 pagesChem 201 Experiment 2 - Lab ReportJohnNo ratings yet
- Lab 5Document15 pagesLab 5NTEYE CHITONGENo ratings yet
- Practical 1 PDFDocument6 pagesPractical 1 PDFlimNo ratings yet
- Practical 1 PDFDocument6 pagesPractical 1 PDFlimNo ratings yet
- Measurement of Rancidity and Peroxide Value in Vegetable OilDocument7 pagesMeasurement of Rancidity and Peroxide Value in Vegetable OilIgotprime ScammedsNo ratings yet
- Lab Report Experiment 3 - Esterification Reactions of VanillinDocument13 pagesLab Report Experiment 3 - Esterification Reactions of VanillinSITI NUR AFIQAH MAHAZANNo ratings yet
- Travoprost: T - L C I T 201 USP Travoprost RSDocument4 pagesTravoprost: T - L C I T 201 USP Travoprost RSShamimaNo ratings yet
- International Junior Science Olympiad Jakarta - Indonesia December 5-14,2004 Solutions ForDocument7 pagesInternational Junior Science Olympiad Jakarta - Indonesia December 5-14,2004 Solutions Foralphamale173No ratings yet
- 3. Fitoterapi Diabetes MellitusDocument28 pages3. Fitoterapi Diabetes MellitusWidya AlamandaNo ratings yet
- Redox TitrationDocument14 pagesRedox Titrationnorsiah100% (2)
- Pepsin Lab (Amelia's) C.S.Document8 pagesPepsin Lab (Amelia's) C.S.jahajaha_svensson60950% (2)
- BejiDocument4 pagesBejiSaswata PradhanNo ratings yet
- Bachelor of Science (Hons) Applied ChemistryDocument20 pagesBachelor of Science (Hons) Applied Chemistryfaiqah hasbullah100% (1)
- (Skt3013) Laboratory Report - Exp 4Document10 pages(Skt3013) Laboratory Report - Exp 4Fiona TiwonNo ratings yet
- Ekstraksi Andre-1Document11 pagesEkstraksi Andre-1Andre YosiNo ratings yet
- Batch Distillation AnalysisDocument16 pagesBatch Distillation AnalysisVivek AgrawalNo ratings yet
- 2023 HCI P4 Mark SchemeDocument8 pages2023 HCI P4 Mark Schemeanya desilvaNo ratings yet
- Characterizing Serine Proteases ActivityDocument8 pagesCharacterizing Serine Proteases ActivityDeniz YılmazNo ratings yet
- Langmuir Adsorption IsothermDocument8 pagesLangmuir Adsorption Isothermsexycassie100% (5)
- Bachelor of Biomedical Science (Hons) Programme Metabolic Biochemistry BBS2121Document6 pagesBachelor of Biomedical Science (Hons) Programme Metabolic Biochemistry BBS2121limNo ratings yet
- Eclr 01Document8 pagesEclr 01Nayanathara PilapitiyaNo ratings yet
- 001L- (AQ) - شيت 1Document22 pages001L- (AQ) - شيت 1Amr OkashaNo ratings yet
- CampomanesETH BSN1C CHY47.1 DS01Document10 pagesCampomanesETH BSN1C CHY47.1 DS01ELSPETH TAMAR CAMPOMANESNo ratings yet
- Hasil Pengamatan Ko AsetanilidaDocument10 pagesHasil Pengamatan Ko AsetanilidaReza MalvinoNo ratings yet
- Title: Experiment 3 Lab ReportDocument6 pagesTitle: Experiment 3 Lab ReportSheikh BajunaidNo ratings yet
- Experiment 18Document5 pagesExperiment 18rhodalyn apiadoNo ratings yet
- Bab 4 Hasil Dan PembahasanDocument3 pagesBab 4 Hasil Dan PembahasanM SyafNo ratings yet
- LAB Report 3Document8 pagesLAB Report 3Phương Vân0% (1)
- Research Methodology ChapterDocument13 pagesResearch Methodology ChapterAmar RazinNo ratings yet
- Extraction of Eugenol From Clove OilDocument8 pagesExtraction of Eugenol From Clove OilLauren MNo ratings yet
- Simultaneous Estimation of Residual Solvents (Ethanol, Acetone, Dichloromethane and Ethyl Acetate) in Dosage Form by GC-HS-FIDDocument7 pagesSimultaneous Estimation of Residual Solvents (Ethanol, Acetone, Dichloromethane and Ethyl Acetate) in Dosage Form by GC-HS-FIDchiralicNo ratings yet
- Lab 6 - QUANTIFICATION OF TOTAL LIPID CONTENT - Vũ Trọng Thức PDFDocument17 pagesLab 6 - QUANTIFICATION OF TOTAL LIPID CONTENT - Vũ Trọng Thức PDFTu HaNo ratings yet
- CHM 260 Lab Report Exp 4Document7 pagesCHM 260 Lab Report Exp 4Warina 01No ratings yet
- Lab 02Document8 pagesLab 02Sakib PkNo ratings yet
- Questions: I (1.5mtr) - I (1mtr) I (1mtr) I (1.5mtr)Document2 pagesQuestions: I (1.5mtr) - I (1mtr) I (1mtr) I (1.5mtr)eyrasyazira94No ratings yet
- Beer Lambert ExDocument11 pagesBeer Lambert ExHarsh DesaiNo ratings yet
- CLS JEEAD-18-19 XI Che Target-1 SET-2 Chapter-1Document32 pagesCLS JEEAD-18-19 XI Che Target-1 SET-2 Chapter-1vishavpreet yadavNo ratings yet
- Total Suspended Solid Volume of Sample Used 50 ML: Data and CalculationDocument6 pagesTotal Suspended Solid Volume of Sample Used 50 ML: Data and CalculationaineenaNo ratings yet
- BAB II Prak AnorDocument5 pagesBAB II Prak AnorLisnaNo ratings yet
- Measuring Acid Concentration Using Potentiometric TitrationDocument4 pagesMeasuring Acid Concentration Using Potentiometric TitrationBanyu IdzharNo ratings yet
- 568Document3 pages568Elen MariNo ratings yet
- SB MBC Ma-1 PDFDocument1 pageSB MBC Ma-1 PDFElen MariNo ratings yet
- POL S 102 Syllabus - Khatuna Chapichadze Modified For 11 Weeks - 2020Document16 pagesPOL S 102 Syllabus - Khatuna Chapichadze Modified For 11 Weeks - 2020Elen MariNo ratings yet
- HomeworkDocument13 pagesHomeworkElen MariNo ratings yet
- POL S 102 Syllabus - Khatuna Chapichadze Modified For 11 Weeks - 2020Document16 pagesPOL S 102 Syllabus - Khatuna Chapichadze Modified For 11 Weeks - 2020Elen MariNo ratings yet
- HomeworkDocument13 pagesHomeworkElen MariNo ratings yet
- Atlas Ci30002Tier-PropanDocument3 pagesAtlas Ci30002Tier-PropanMarkus JeremiaNo ratings yet
- Greek Myth WebquestDocument9 pagesGreek Myth Webquesthollyhock27No ratings yet
- DaburDocument3 pagesDaburchiru94No ratings yet
- Bareos Manual Main ReferenceDocument491 pagesBareos Manual Main ReferenceAlejandro GonzalezNo ratings yet
- Fellowship in OncotherapeutDocument3 pagesFellowship in OncotherapeutNayan ChaudhariNo ratings yet
- Map Project Rubric 2018Document2 pagesMap Project Rubric 2018api-292774341No ratings yet
- Internship Report Zannatul Ferdousi Alam YameemDocument51 pagesInternship Report Zannatul Ferdousi Alam YameemZannatul Ferdousi Alam YameemNo ratings yet
- Addressable Fire Detection and Control Miniplex TranspondersDocument8 pagesAddressable Fire Detection and Control Miniplex TranspondersAfdhal SyahrullahNo ratings yet
- HEC-HMS Tutorials and Guides-V3-20210529 - 140315Document756 pagesHEC-HMS Tutorials and Guides-V3-20210529 - 140315Ervin PumaNo ratings yet
- CP302 Example 01 OKDocument5 pagesCP302 Example 01 OKAw Yeong Pei Yee100% (1)
- 01 - PV - RESCO 1d Workshop - S1 PDFDocument61 pages01 - PV - RESCO 1d Workshop - S1 PDFDeasy KurniawatiNo ratings yet
- GSAA HET 2005-15, Tranche B2 / BSABS 2005-TC2, Tranche M6 Shown As An Asset of Maiden LaneDocument122 pagesGSAA HET 2005-15, Tranche B2 / BSABS 2005-TC2, Tranche M6 Shown As An Asset of Maiden LaneTim BryantNo ratings yet
- JMPRTraininga I5545e PDFDocument500 pagesJMPRTraininga I5545e PDFmvptoxNo ratings yet
- Reporte Corporativo de Louis Dreyfus Company (LDC)Document21 pagesReporte Corporativo de Louis Dreyfus Company (LDC)OjoPúblico Periodismo de InvestigaciónNo ratings yet
- Armv8-A Instruction Set ArchitectureDocument39 pagesArmv8-A Instruction Set ArchitectureraygarnerNo ratings yet
- Grab E-Receipt for 15,000 RP Ride on March 30Document1 pageGrab E-Receipt for 15,000 RP Ride on March 30WellyNo ratings yet
- DOJ OIG Issues 'Fast and Furious' ReportDocument512 pagesDOJ OIG Issues 'Fast and Furious' ReportFoxNewsInsiderNo ratings yet
- A New Aftercooler Is Used On Certain C9 Marine Engines (1063)Document3 pagesA New Aftercooler Is Used On Certain C9 Marine Engines (1063)TASHKEELNo ratings yet
- Etherpad Text-Based TutorialDocument5 pagesEtherpad Text-Based Tutorialapi-437836861No ratings yet
- Why it's important to guard your free timeDocument2 pagesWhy it's important to guard your free timeLaura Camila Garzón Cantor100% (1)
- Textbook of Heat Transfer Sukhatme S PDocument122 pagesTextbook of Heat Transfer Sukhatme S PSamer HouzaynNo ratings yet
- BED 101 Voc & Tech. Course ContentDocument3 pagesBED 101 Voc & Tech. Course ContentSunday PaulNo ratings yet
- Tax1 Course Syllabus BreakdownDocument15 pagesTax1 Course Syllabus BreakdownnayhrbNo ratings yet
- A Rail-To-Rail Constant Gain Buffered Op-Amp For Real Time Video ApplicationsDocument8 pagesA Rail-To-Rail Constant Gain Buffered Op-Amp For Real Time Video Applicationskvpk_vlsiNo ratings yet
- 1.1 Enterprise AssessmentDocument1 page1.1 Enterprise AssessmentGermanRobertoFongNo ratings yet
- Idler Sprockets: Poly Chain GT and Powergrip GTDocument2 pagesIdler Sprockets: Poly Chain GT and Powergrip GTVolodymуr VorobetsNo ratings yet
- Inspection and Test Plan Steel Sheet Pile DriDocument6 pagesInspection and Test Plan Steel Sheet Pile DriSofda Imela100% (1)
- Basic Facts in EventDocument1 pageBasic Facts in EventAllan AgpaloNo ratings yet
- Rg213 Rgflex Coax Braided Cable: Product Data Sheet RG213-50JFDocument1 pageRg213 Rgflex Coax Braided Cable: Product Data Sheet RG213-50JFPancho BerríosNo ratings yet
- SocorexDocument6 pagesSocorexTedosNo ratings yet