Professional Documents
Culture Documents
Total Body Water (TBW) - 60% of Total Body Weight: Do Not Distribute © Rat
Uploaded by
Arlie Baluca0 ratings0% found this document useful (0 votes)
33 views17 pagesOriginal Title
5_6064403571804209236.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
33 views17 pagesTotal Body Water (TBW) - 60% of Total Body Weight: Do Not Distribute © Rat
Uploaded by
Arlie BalucaCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 17
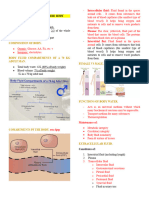
INTRACELLULAR FLUID (ICF) – fluid inside the cells
EXTRACELLULAR FLUID (ECF) – occupies extracellular
Plasma and interstitial fluid = same composition
compartment
- Major difference is the absence of plasma
CELL MEMBRANE – barrier that separates these two
proteins from interstitium
THE INTRACELLULAR AND EXTRACELLULAR FLUIDS
- Plasma proteins affect solute distribution
Total Body water is the sum of ICF and ECF volumes
because of their VOLUME and ELECTRICAL
Total Body water (TBW) – ~60% of total body weight
CHARGE
(males);
Volume Occupied by Plasma Proteins
~50% total body weight
Proteins (and lipids) occupy ~7% of total plasma
(females)
volume
~65-75% in an infant
Plasma composition of ions is reported in units
Variability in amount of adipose tissue can influence the
of milliequivalents (meq) per liter of plasma
fraction
solution
Acute changes detected simply by monitoring the body
Milliequivalents per liter of protein-free plasma
weight
solution – more meaningful unit for cells bathed
Assuming 70kg male has ~42L TBW:
in interstitial fluid
- 60% (25L) is intracellular
o Because only protein-free portion of
- 40% (17L) is extracellular
plasma can equilibrate across capillary
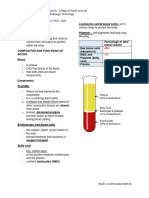
Plasma Volume
wall (PLASMA WATER)
20% of ECF (~3L)
Plasma water fraction is 93%
Blood volume – total volume of intravascular
Effect of Protein Charge
component (6L)
Plasma proteins carry net negative charge
Plasma volume 3L
Tend to retain cations in plasma
Cell elements of blood 3L
Cation concentration in protein-free solution is
o Hematocrit – Fraction of blood occupied by
lower by ~5%
these cells
Anion concentration of protein-free solution is
Interstitial fluid
higher by ~5%
75% of ECF
All body fluids have approximately the same
Bathes non-blood cells of the body
osmolality, and each fluid has equal number of
Two smaller compartments communicate only
positive and negative charges
slowly:
Osmolality
o Dense connective tissue
- All cell compartments have approximately the
o Bone matrix
same osmolality
Capillaries – barrier that separates intravascular
- Describes the total concentration of all particles
and interstitial compartments
that are free in a solution
Transcellular fluids
o Particles bound to macromolecules do
5% (~1L)
not contribute
Space surrounded by epithelial cells
- Expressed as the number of osmotically active
Includes synovial fluid and CSF
particles per kilogram of water
ICF is rich in K+, whereas ECF is rich in Na+ and Cl-
- Plasma proteins contribute: ~14mEq/L
Cell maintains relatively high K+ concentration and low
o Measured in grams per liter is very low
Na+ by using Na-K pump
o Contribute only slightly to total
- Actively extrudes Na
osmolality (~1 mOsm)
- Transports K actively into cell
- Total solute concentration of the intracellular
Transcellular fluids differ greatly in composition from
compartment higher than in interstitium
each other and from the plasma because they are
o Considerable fraction of ions is bound
secreted by different epithelia
DO NOT DISTRIBUTE © RAT
1
to proteins or complexted to other o Steady state – both rate of transport
small solutes and driving force are constant with time
o Proteins themselves attach to materials Net transport only takes place when net driving
that are out of solution force is displaced from equilibrium
o Mg and Ca are either complexed or At equilibrium, the chemical and electrical potential
bound energy differences across the membrane are equal but
Electroneutrality opposite
- All solutions must respect principle of BULK [𝑋]𝑖
∆𝜇𝑥 = 𝑅𝑇𝑙𝑛 + 𝑧𝑥 𝐹(𝜑𝑖 − 𝜑𝑜)
NEUTRALITY [𝑋]𝑜
- The excess positive charge is balanced out by First term on right hand side – difference in potential
intracellular macromolecules as well as organic energy
phosphates - Energy change as X moves across the
- Anion gap – usually 9-14mEq/L membrane
o Difference between ignored anions and Second term – difference in electrical potential energy
ignored cations - Energy change as a mole of charge particle
SOLUTE TRANSPORT ACROSS CELL MEMBRANES moves across the membrane
In passive, noncoupled transport across a permeable - (𝜑𝑖 − 𝜑𝑜)- Voltage difference across the
membrane, a solute moves down its electrochemical membrane OR Vm/membrane potential
gradient - X is at equilibrium if electrochemical potential
A substance can passively move across a energy difference is zero
membrane when there is both a favorable - ∆𝜇𝑥 - net driving force (unit: joules/mole)
driving force and an open pathway - Two special cases of equilibrium:
Permeable – when a pathway exists o When eiehter chemical or electrical
Driving force determines passive transport term is zero the other must also be zero
Electrochemical gradient – energy difference o When neither chemical nor electrical is
acting on a solute between two compartments zero, equilibrium can occur only when
Electrochemical potential energy difference the two terms are equal but of opposite
includes concentration of concentration sign
(chemical), and voltage(electrical)
Noncoupled transport – movement of X not 𝑅𝑇 [𝑋]𝑖
directly coupled to the movement of any other 𝑉𝑚 = 𝐸𝑥 = − 𝑙𝑛
𝑍𝑥 𝐹 [𝑋]𝑜
solute or to any chemical reaction - NERNST equation – describes the conditions
Concentration gradient will act as a driving when an ion is in equilibrium
force but voltage difference will also drive net - X can be in equilibrium only if voltage difference
movement of X across the membrane equals Equilibrium
Electrochemical gradient for X is the only potential (EX)
driving force that contributes to transport of X - EX – value that membrane voltage would have
Must always be “downhill” to have for X to be in equilibrium
Unidirectional flux – movement of X across the (Vm – EX) is the net electrochemical driving force acting
membrane in one direction on an ion
Net flux – algebraic sum of the two - When net driving force is negative – cations will
unidirectional flux (or net transport rate) enter the cell and anions will exit
o Only occurs if unidirectional fluxes are o When Vm is more negative than EX (cell
unequal is too negative for X to be in
Equilibrium – when no net driving force is acting equilibrium), a cation will tend to enter
on X the cell
o A special case of steady state wgherein In simple diffusion, the flux of an uncharged substance
there is no net driving force thus no net through membrane lipid is directly proportional to its
transport concentration difference
DO NOT DISTRIBUTE © RAT 2
- Only factor that determines the direction of net transmembrane path
transport is the driving force a. Two gates never open at the same time
- Kinetics – the rate of transport b. If two gates are both closed, transiting
o Requires knowing actual mechanism particles is trapped, or OCCLUDED
that mediates passive X transport Water-filled pores can allow molecules, some as large
- Flux (JX) – how fast X moves as 45 kDa, to corss membrane passively
o Units of moles/(square Pores – provide aqueous transmembrane
centimeterxsecond) conduit
- Partition coefficient – how fast X can dissolve in Porins – large-size pores
membrane lipid Mitochondrial porin allows solutes as large as 5
- Diffusion coefficient – how fast X moves once it kDa to diffuse passively
is in the membrane One mechanism cytotoxic T lymphocytes kill
- Membrane thickness – Distance to traverse is target cells release monomers of pore-
short forming protein called PERFORIN
- Permeability coefficient of X (PX) – combining o Perforin assemble like staves of a barrel
above three factors to form doughnut-like channels (16nm
- Fick’s Law diameter)
𝐽𝑥 = 𝑃𝑥 ([𝑋]𝑜 − [𝑋]𝑖) Binding of antibodies (“classic” pathway) or
- Net flux – difference between unidirectional presence of native polysaccharides
fluxes (“alternative” pathway) triggers complement
- Unidirectional influx is proportional to outside cascade
concentration o Doughnut-like structure with diameter
- Unidirectional efflux is proportional to outside of 10nm; monomers of C9
concentration Nuclear pore complex (NPC) – 30 different
- Next flux is proportional to concentration proteins
difference o Molecular mass of 108 Da; Outer
- Kinetic behavior of transport system cannot diameter of 100nm
violate laws of energetic or thermodynamics o Involves ATP hydrolysis but also has
- When concentration gradient for neutral passive component
substance is zero, the flux is zero o Contained within NPC is simple aqueous
- Law of simple diffusion: when concentration pore with diameter of ~9nm
gradient for neutral substance is zero, flux is Allows molecules <45 kDa but
zero completely restricts proteins
Some substances cross the membrane passively >60kDa
through intrinsic membrane proteins that can form Aquaporin 1 (AQPI) – first water channel to be
pores, channels, or carriers studied
Pathways are formed from integral proteins; three o 28 kDa protein; belong to larger family
types: of aquaporins (AQP)
1. Pore – always opens o Exist as tetramers in lipid bilayer
a. Porins in outer membrane of Each monomer = six membrane
mitochondria spanning helices + two shorter
b. Aquaporin water channels helices that dip into plane of
2. Channel – alternately open and closed; equippd membrane
with movable barrier or gate Gated channels, which alternately open and close,
a. Virtually all ion channels allow ions to cross the membrane passively
b. Gating – process of opening and closing Gated ion channels = one or more polypeptide
of the barrier subunits with a-helical spanning membrane
c. Channel is a gated pore segments
3. Carrier – never offers continuous Functional components:
DO NOT DISTRIBUTE © RAT 3
o Gate – determines whether channel is Move H+ into the
open or closed; each state reflecting cells (normally);
different conformation Hv1 channel tend
o Sensors – respond to changes in to be closed
membrane voltage, second-messenger normally;
Mediates H+ extrusion
systems, or ligands (neurohomal Proton Activates when:
during states of strong
agonists) channel membrane
membrane
Regulates transitions b/n open (Hv1 H+ depolarizes, or
depolarization, or severe
and closed states channel) cytoplasm
intracellular acidification
o Selectivity filter – determines the acidifies (when
classes of ions or particular ions that driving force
have access favors
o Open channel pore – provides a OUTWARD
continuous pathway b/n two sides movement)
During each channel opening, many ions flow = Anion
Modestly Transepithelial
sufficient to be detected as small current channels
negative (move movement of Cl from
(Vm – EX) (Cl- or
out) lumen to blood
Channel Electrochemical Physiologic functions HCO3-)
driving force
(1) Transmission of Some carriers facilitate the passive diffusion of small
information – solutes such as glucose
responsible for action Carrier-mediated transport systems
Strongly negative
Na+ potential - Specific affinity for binding one or a small
(large INWARDLY
channel (2) Renal tubule and number of solutes
directed)
intestine – ENaC Na+; - Simplest – mediates facilitated diffusion
allow Na to enter - Cotransporters – carry two or more solutes in
epithelial cell the same direction
Fairly close to - Exchangers – move solutes in opposite
zero or Generates resting directions
somewhat membrane voltage that Solute Carrier (SLC) Superfamily – all carriers that do not
K+
POSITIVE is inside negative; help hydrolyze ATP or couple to an electron transport chain
channel
(equilibrium or terminate action - 52 SLC families contains up to 53 genes
moves OUT of potentials - Differ in:
cell) o molecular mechanism
Transmembrane o kinetic properties,
signaling for both o regulation,
Strongly negative
Ca2+ excitable and o sites of membrane targeting,
(tends to move
channel nonexcitable cells; o tissues in which they are expressed, or
INTO the cell)
generating action o developmental stage they are
potential in some cells expressed
Carrier-mediated transport behave according to a
general kinetic scheme for facilitated diffusion in a cycle
of SIX steps
- Can only mediate downhill, passive transport of
X
- Mediates only facilitated diffusion
- Fixed number of carriers is available and they
have limited speed
- If extracellular X concentration is increased,
DO NOT DISTRIBUTE © RAT 4
influx of X will eventually reach a maximal value o Carrier – complete cycle of
once carriers have become loaded conformational changes
- Simple diffusion – no maximal rate of transport Transports only small, fixed
- Relationship describing carrier-mediated number
transport follows MICHAELIS-MENTEN kinetics Mediates COUPLED modes of
as do enzymes transport
o Velocity of enzymatic reaction depends The Na-K pump, the most important primary active
on substrate concentration, Michaelis transporter in animal cells, uses the energy of ATP to
constant, and maximal velocity extrude Na and take up K
o The lower the Km – the higher the Active transport – can transfer solute uphill
AFFINITIY of the transporter for the Primary Active transport – driving force comes from
solute (transporters are easily filled favorable energy change; associated with exergonic
because they bind easily) chemical reactions (ATP hydrolysis)
- Ionophores – “ion carriers”; bind to an ion on Pumps – all energized by ATP hydrolysis hence,
one side, diffuse across lipid phase, and release are ATPases
ion on the opposite side Secondary Active transport – driving force provided by
o Valinomycin – K ionophore coupling uphill movement to the downhill movement of
- GLUT glucose transporters – members of SLC2 one or more solutes
family Na-K pump – first enzyme recognized to be an ion pump
o 12 membrane spanning segments Has α and β subunits
o Multiple hydrophilic polypeptides loop o α – 10 transmembrane segments;
facing ECF or the ICF – form a catalytic subunit; mediates active
permeation pathway through lipid transport
bilayer o β – 1 transmembrane segment;
7, 8, 11 spanning segments essential for proper assembly and
Also acts as gates and solute- membrane targeting of the Na-K pump
binding sites Couples extrusion of 3 Na for uptake of 2 K ions
- GLUT1 – expressed constitutively on the surface with hydrolysis of 1 ATP molecule
- GLUT4 – predominantly present in membranes Transport steps of Na-K pump are energetically
of intracellular vesicles (storage pool for uphill
transporters) o Can be reversed and forced to
o Insulin recruits GLUT4 isoform to the synthesized ATP
plasma membrane from storage pool o ATP releases so much energy – brings
- Urea transporter (UT) – SLC14 family about net active exchange for Na for K
- Organic cation transporter – SLC22 family in desired directions
o Electrogenic – moves an electrical ONLY PRIMARY ACTIVE TRANSPORT FOR Na
charge (or carries current) MOST IMPORTANT PRIMARY ACTIVE
The physical structures of pores, channels, and carriers TRANSPORT FOR K
are quite similar Restricted to basolateral side of epithelial cells
All have segments surrounding a solute Responsible for maintaining a low [Na]I and a
permeation pathway high [K]I relative to ECF
Differ kinetically Exists in two major conformational states:
o Pore – continuously open o E1 – binding sites for the ions face
o Channel – undergo conformational inside of cells
transitions between closed and open o E2 – binding sites face the outside
states Member of E1-E2 ATPases OR P-type ATPases
When open – open to both ECF Ordered cycling that underlies action of the
and ICF simultaneously pump
DO NOT DISTRIBUTE © RAT 5
Pores Channels Carriers
+
Example Water channel (AQP1) Shaker K channel Glucose transporter (GLUT1)
Conduit through membrane Always open Intermittently open Never open
None (continuously Cycle of conformational
Unitary event Opening
open) change
Particles translocated per
---- 6x104 1-5
event
Particles translocated per
Up to 2x109 106-109 when open 200-50,000
second
Eight stages of Na-K pump:
Stage 1: ATP-bound E1 * ATP state – just after the
pump has released its bound K+ to the ICF; Na+
binding sites have high affinities for Na+
Stage 5: Empty E2-P state – 3 bound Na ions dissociate
protein undergoes MINOR change to empty
E2-P form; HIGH affinity binding for
extracellular K
Stage 2: Na-bound E1 * ATP * 3Na state – Three Na
ions bind
Stage 6: K-bound E2-P * 2K state – two K ions bind
Stage 3: Occluded E1-P * (3NA) state – ATP previously
bound phosphorylates the pump at an
aspartate redisue triggers MINOR change in
E1 form now occludes 3 bound Na ions
INACCESIBLE TO BOTH ICF and ECF; ADP leaves
Stage 7: Occluded E2 * 2K state – hydrolysis of
acylphos-phate bond releases inorganic
phosphate minor change happens pump
occludes the two bound K ions
Stage 4: Deoccluded E2-P * 3Na state – MAJOR change
shifts pump from E1 to E2 conformation; has
two effects:
o Pump now communicates to ECF
Stage 8: Deoccluded E1*ATP*2K state – Binding of ATP
o Affinity for Na decreases causes MAJOR change shifts pump from E2
back to E1. Change has two effects:
o Pump now communicates with ICF
o Affinity for K decreases
DO NOT DISTRIBUTE © RAT 6
Coppe
r Mutated in
pump Wilson’s
P1B
(ATP7 disease
B)
Stage 1: ATP-bound E1*ATP state – returns the pump to *Plasma-membrane Ca ATPase
its original state ** SERCAS – Sarcoplasmic and endoplasmic reticulum
calcium ATPases
Stoichiometry of the pump – 3 Na to 2 K ***Along with Na-K pump, only member of P2C
Each cycle – next extrusion of one positive subfamily with B subunit
charge from the cell The F-type and V-type ATPases transport H+
o ELECTROGENIC F-type or FoF1 ATPases
Rate of active transport – saturable function Of the inner membrane mitochondria
o Number of pumps is finite Final step in ATP synthesis pathway
o Also saturable function of ATP Like a lollipop held in your hand
Depends on metabolic rate of o Hand-like: Fo portion embedded in the
cell membrane
HALLMARK of Na-K pump – blocked by cardiac Pathway for H transport
glycosides (Ouabain and digoxin) Three subunits (a, b, c)
o Compounds have high affinity for E2-P ab2c10-12
state which also have high affinity for o F1: points into mitochondrial matrix
extracellular K “Stick” – γ subunit with
Binding of K competitively attached ε subunit
antagonized “Candy” – has ATPase activity
o Hypokalemia potentiates digitalis Three alternating pairs
toxicity in patients of a and Bs ubunits
Besides the Na-K pump, other P-type ATPases include δ subunit
H-K and Ca pump α3β3γδε
Family of P-type ATPases share significant similarity o Molecular mass: 500 kDa
with alpha subunit The ‘hand’ acts as a turbine that rotates in the
Structu plane of the membrane driven by H ions that
Localizati Stoichiom
Pump re/ Regulation flow through the turbine
on etry
Family Stick is an axle (Y and E subunits of F1) rotates
Parietal with turbine
cells of Phosphoryl Stationary chemical factory is energized by
Extrusion A and
gastric ation rotating axle
of H, ***B
H-K
gland;
uptake of subunit
through E1 o One ATP synthesized for 120o turn of
Kidney and E2 turbine
K (2:2); s;
and intermediat The Fo a and b subunit and δ – stator that holds
1 ATP P2C
intestine es candy in place
s Under physiologic conditions – F-type ATPases runs as
Most an ATP synthase
cells;
1 H: 1 Ca: Citric acid cycle captures energy as electrons
sarcoplas and transfer these to NAD and FAD
Ca 1 ATP P2A
(*PM
mic
**SERCAS subfam == NADH and FADH2 transfers electrons to ETC
CA)
reticulu
- 2 H: ily As electrons are transferred, electrons gradually
m in lose energy
2Ca: 1ATP
muscle o Finally combine with 2H and ½ O2 to
cells form H2O
Complex I, III, IV pump H across the inner
DO NOT DISTRIBUTE © RAT 7
membrane glycoprotein) cationic DE-
o Established large out-to-in H gradient metabolite BINDING
across the membrane s and DOMAIN
Complex V (FoF1 ATPase) use this large drugs; (NBD)
electrochemical potential energy Pumps binds ATP
o H ions flow backward through FoF1 anticancer
ATPase drugs out
Generates ATP in matrix space of cell
from ADP and Pi 170 kDa
o Chemisomotic hypothesis
o One ATP for every three H+ 2
Will function as ATPase in reverse membrane
V-type H pump -spanning
Membranes surrounding organelles contain domain
vacuolar-type (or V-type) H-ATPase (MSD1 and
o Pumps H from cytoplasm to the interior MSD2)
MRP/CFTR
of the organelle composed
Subfamily
Important for sorting proteins, dissociating Apical of 6
(cystic Low
ligands, optimizing activity of acid hydrolases, membrane membrane
fibrosis conductanc
accumulating neurotransmitters in vesicles of spanning
transmembra e Cl
Apical membranes of renal tubule cells also epithelial segments;
ne channel
have V-type H pumps cells 2 NBDs
conductance
o Independent of K
regulator)
o Similar to hand-held lollipop Cytoplasmi
V-type pump has only six subunits but each c
twice as large as a c subunit in F-type ATPase regulatory
ATP-binding cassette transporters can act as pumps, domain (R)
channels, or regulators separates
Subfamilies named ABCA through ABCG two halves
Some are pumps that hydrolyze ATP to provide for of CFTR
solute transport, but some do not couple energy to
perform active transport CFTR
Location Function Structure - R domain contains multiple PKA and PKC
Mediates phosphorylation sites
efflux of - Neurohumoral agents phosphorylate sites
ABCA Macrophag
phospholipi -- promotes activation of CFTR
Subfamily es
ds and - Binding of ATP to NBDs also controls opening
cholesterol and closing of the channel
Multidrug- ATPases 6 - Two regulatory mechanisms: Protein
resistance and 1o membrane phosphorylation and interaction with NBDs
Liver,
transporters active -spanning
kidney, GIT
(MDRs) transporter and
MDR1 (p- Extrudes NUCLEOTI
Cotransporters, one class of secondary active - Two major classes:
transporters, are generally driven by energy of the o Cotransporters or symporters – move
inwardly directed Na gradient the driving solute and driven solute in
2o transporters can move a solute uphill the same direction
- Fuel it by coupling to downhill movement o Exchangers or antiporters
Location Family Structure Stoichiometry Driving force Function
DO NOT DISTRIBUTE © RAT 8
Inwardly
directed Na
can drive
uphill
accumulation
Useful for
Apical Single subunit 1 Na: 1 of glucose into
DM type 2:
Na- membrane of with 14 Glucose the cell
Reduce
GlucoseCotransporter proximal tubule SLC5 membrane- SGLT1 isoform:
glucose
(SGLT) and small spanning 2 Na: 1 10:1 Na con’cn
reabsorption
intestine segments Glucose gradient = 10-
in kidney
fold glucose
gradient
-60mV=
another 10-
fold
Na+-Driven
Cotransporters for
Organic Solutes
Na-driven amino-acid SLC 6
Proximal tubule --- ---- ---- ----
transporters SLC 38
and small
Na-coupled
intestines
cotransporters for
monocarboxylates, SLC5 ---- ---- ---- ----
dicarboxylates, and
tricarboxylates
Electrogenic
HCO3 efflux Acid-base
NBCS
(into the transporters;
1:3 (Na:HCO3)
Basolateral blood) regulation of
Na/HCO3
membrane of intracellular
Cotransporters SLC4 1:2
certain HCO3 influx pH, for
(NBCs)
epithelial cells epithelial
Electroneutral
HCO3-
NBCs
HCO3 influx secretions
1:1
Na=-Driven Cotransporter of Other Inorganic Anions
Na/I cotransporter SLC5A5
Sulfate cotransporter SLC13
SLC17,
Inorganic phosphate
SLC20,
cotransporter (NaPi)
SLC34
DO NOT DISTRIBUTE © RAT 9
NKCC1:
nonepithelial Inhibited by
cells, furosemide
basolateral and
Na/K/CL membrane of Inwardly umetanide
cotransporter (NKCC) some epithelial directed Na to (loop
Or Bumetanide- cells SLC12 drive diuretics) –
sensitive accumulation increase
cotransporter (BSC) NKCC2: apical of Cl and K urine flow by
membranes of inhibiting
cells in thick transport at
ascending loop LoH
of Henle
Na/Cl Cotransporter Apical
Blocked by
(NCC) or Thiazide- membrane of
SLC12A3 K independent thiazide
sensitive dital tubule of
diuretics
cotransporter kidney
H-driven Cotransporters Driven by downhill inward movement of H
Uptake of
Renal proximal
H/Oligopeptide small
tubule and SLC15
cotransporter (PepT1) peptides;
small intestine
Electrogenic
H-driven amino-acid
SLC36
cotransporters (PAT1)
Moves
Either net
lactate out of
inward or net
cells that
Monocarboxylate outward
produce
cotransporters direction
lactate and
(MCT1) depending on
into cells that
lactate and H
consume
gradient
lactate
Couples influx
Kidney and of H to influx
Divalent metal-ion proximal of Ferrous iron
SLC11
cotransporter (DMT1) portions of or other
small intestines divalent
metals
Exchangers, and another class of secondary active transporters, exchange ions for one another
Transporters exchange cations for cations and anions for anions
Location Family Stoichiometry Driving Force Function
Helps maintain
Inwardly directed steep, inwardly
3 Na: 1 Ca
Na-Ca Exchanger Na drives uphill directed
Ubiquitous SLC8
(NCX) extrusion of Ca electrochemical
Electrogenic
from cell potential energy
difference for Ca
DO NOT DISTRIBUTE © RAT 10
pH regulation and
Almost every cell
Inwardly directed cell volume
Na-H Exchanger Na drives uphill
NHE3: apical SLC9 1:1
(NHE) extrusion os H from NHE3: acid
membranes of
cell; raises pH secretion and Na
several epithelia
absorption
Multidrug and Renal proximal Uptake of H in
toxin extrusion tubule cells, SLC47 exchange for
transporter (MATE) hepatocytes organic cations
Inwardly directed pH regulation
1 Na: 2 HCO3 (in
Na-driven Cl-HCO3 Na to drive uphill (keeps it alkaline);
SLC4 one direction): 1 Cl
Exchanger (NDCBE) entry of HCO3 into also Na
(opposite direction)
cell reabsorption
AE1: transport
All cells express
HCO3 into RBC in
one of the three
the lung and out of
electroneutral SLC Function
lung in peripheral
4 CL-HCO3 independently of
tissues
exchangers – anion Na
SLC4 or
Cl-HCO3 exchanger exchangers (AE1 to
SLC26 AE2 and AE3
AE3) Inwardly directed
(acidifies cells)
Cl drives HCO3 out
SLC26 family: can of cell
Uptake of Cl =
be electrogenic
regulation of cell
(not 1:1)
volume
Other Anion Exchangers
Cl-formate Secondary active
Renal proximal
exchange and Cl- SLC26A6 uptake of Cl and
tubule
oxalate exchange secretion of oxalate
Transport I (for
Pendrin
thyroid gland)
Organifc anion- Mediate uptake of
transporting bile acids, bilirubin,
SLC21
polypeptides and test substrate
(OATPs) bromosulphthalein
Prostaglandin Mediates uptake of
SLC21
transporter (PGT) prostanoids
Mediate uptake of
endogenous
organic anions,
Organic anion
SLC22 drugs, penicillin,
transporters (OATs)
and p-
aminohippurate
(test substrate)
Renal proximal Mediates urate
URAT1 SLC22
tubule transport
REGULATION OF INTRACELLULAR ION high
CONCENTRATIONS - Sodium – 145 mM
The Na-K pump keeps [Na+] inside the cell low and [K+] o predominant cation in ECF
DO NOT DISTRIBUTE © RAT 11
o Maintained by Na-K pump - Sarcoplasmic reticulum and endoplasmic
- Potassium – 4.5 mM reticulum – actively sequester cytosolic Ca in
o Predominant cation in ICF intracellular stores
- Oubain – inhibits Na-K pump - Stores of Ca released in bursts as part of signal-
- -60 mV – inside-negative membrane voltage transduction process
generated by Na-K pump - There is a limit to how Ca a cell can store;
- Electrogenic pump – causes a net outward extrusion must balance passive influx of
current of a positive charge Calcium
o Generates an inside negative Vm Ca pump (PMCA) on the plasma membrane
o Small contribution to negative Vm - Most cells contain Ca pump – major role in
- Active K accumulation favors the exit of K extruding Calcium
through K channels - Rising levels of intracellular Ca stimulate Ca
o Tendency of K to exit (with unmatched pump to extrude Ca
negative charges left behind) is the - Pump itself is incapable of feedback control –
MAIN cause of inside-negative has a high Km for Clacium (virtually inactive at
membrane voltage physiological Ca)
- Ba2+ - inhibitor of K channels o High levels of Ca bind to calmodulin
o Vm becomes less negative (cell o Ca-CaM binds to Ca pump lowers Km
depolarizes) stimulates Ca extrusion
- Principal pathway for current flow is through K o Low levels of Ca Ca-Cam dissociates
channels from Calcium pump
- Inside-negative Vm + large concentration - At levels of ~100nM, Ca pump is major route of
gradient for Na = strongly favors passive entry Ca extrusion
of Na Na-Ca Exchanger (NCX) on the Plasma Membrane
- Cell harness the energy of Na entry for three - Extrudes Clacium only when [Ca]I rises
major purposes: substantially above normal levels
o Amiloride-sensitive Na channels (ENaCs) - Restores low Ca when large influxes of Ca occur
– restricted to apical or luminal surface; - Most notable in excitable cells such as neurons
Na-K pump restricted to basolateral and cardiac muscle
Transepithelial Na transport In most cells, [Cl-] is modestly above equilibrium
takes place because Cl uptake by Cl-HCO3 exchanger and Na/K/Cl
o Excitable cells – passive Na entry cotransporter balances passive Cl efflux through
occurs; role in generation of action channels
potential - Cl ICF< Cl ECF
Na is cycled at high energy cost - All cells have ANION-SELECTIVE CHANNELS – Cl
for information transfer can permeate passively
o Every cell – to drive the secondary - Cl-HCO3 exchanger – Most common pathway
active transport of nutrient and ions for Chloride uptake
The Ca pump and the Na-Ca exchanger keep o Outwardly directed energy for HCO3 –
intracellular [Ca2+] four orders of magnitude lower driving force for entry of Cl
than extracellular [Ca2+] - Na/K/Cl cotransporter – stimulated by low Cl;
- Calcium - ~1mM in ECF but 100 nM in ICF uphill
- Negative membrane voltage and large chemical - Passive Cl efflux through anion-selective
gradient – inwardly directed electrochemical channels opposes Cl uptake mechanisms
gradient for Calcium FAR LARGER THAN ANY - K/Cl cotransporter – another factor; driven by
OTHER ION outward K gradient; moves Cl out of cell too
- Calcium channels gated by voltage or by o K gradient promotes Cl EFFLUX by
humoral agents generating negative Vm and by driving
o Rapid entry occurs only in short bursts K/Cl cotransport
- What transport mechanism keep Calcium low? The Na-H exchanger and Na-driven HCO3 transporters
Ca Pump (SERCA) in Organelle Membranes keep the intracellular pH and HCO3 above their
DO NOT DISTRIBUTE © RAT 12
equilibrium values - AQPs – increase membrane H2O permeability
Extracellular pH ~7.4 - Erythrocytes and renal proximal tubule – AQP1
- HCO3 ~24mm always present
- PCO2 ~40mm Hg - Collecting duct regulate H2O permeability by
Intracellular pH ~7.2 inserting AQP2 channels under control of
- pHi – actually much more alkaline if H and arginine vasopressin
HCO3 were passively distributed across the cell - Nonsaturable function
membrane - Two passive driving force:
- H can enter and HCO3 can exit passively o Chemical potential energy difference –
o Both occur at low rate difference in water concentration
o H – cation channels and H selective o Energy difference per mole of H2O –
channels results from difference in hydrostatic
o HCO3 – through most Cl channels pressure
- -60mV – 10-fold concentration gradient for H - At equilibrium osmotic pressure difference is
o 10-fold higher within cell – corresponds equal to hydrostatic pressure difference
to pH that is 1 pHi more acidic than pHo - Animal cells cannot tolerate any significant
- Acid extruders – transport acid out of cell; hydrostatic pressure difference w/o derofrming
functions in acid extrusion o Thus it is always near zero = no
o Secondary active transporters significant driving force
energized by Na gradient - Movement of H2O out of cells driven by
Na-H, Na-driven Cl-HCO3 ex- osmotic gradients only
changers, Na/HCO3 - Osmosis – movement of H2O driven by osmotic
cotransporters – most gradient
important - Hydrostatic pressure differences – important for
Na/HCO3 – 1:1 or 1:2 driving fluid out of across the walls of capillaries
o Sensitive to changes in pH – stimulated - Presence of greater concentration of proteins
when cell is acidified and inhibited sets up difference in osmotic pressure that pull
when cell is alkalinized fluid back into capillary
o V-type, H pumps or H-k pumps on o Called COLLOID OSMOTIC PRESSURE or
collecting duct and the stomach ONCOTIC PRESSURE
- Because of acid extruders pHi is not far more - Hydrostatic pressure = Oncotic pressure
alkaline than 7.2 equilibrium
o Acid loaders balance acid extrusion - Hydrostatic > oncotic = ULTRAFILTRATION
- Passive leakage of H and HCO3 through (movement ouf ot capillary)
channels tend to acidify cells Because of the present of impermeant charge proteins
- Transporters move HCO3 out of cells within the cell, Donnan forces will lead to cell swelling
o Cl-HCO3 exchanger – most common - Relative exclusion of NaCl from intracellular
o Electrogenic Na/HCO3 cotransporter space is vital for maintaining H2O content
(1:3) – moves HCO3 across renal - No Na-K pumps = cells swell
proximal tubules - Gibbs-Donnan equilibrium
WATER TRANSPORT AND THE REGULATION OF CELL [𝑁𝑎]𝑜 [𝐶𝑙]𝑖
= =𝑟
VOLUME [𝑁𝑎]𝑖 [𝐶𝑙]𝑜
Water transport is driven by osmotic and hydrostatic - r = donnan ratio
pressure differences across membranes - Total number of positive charges must balance
- H2O molecules can dissolve in lipid bilayers at total number of negative charges (bulk
low but finite rate by simple diffusion electroneutrality)
o Ease of diffusion depends on lipid The Na-K pump maintains cell volume by doing
composition osmotic work that counteracts the passive Donnan
o Low fluidity membrane (PLUS forces
cholesterol) = further reduce H2O - Distribution of ions toward Donnan equilibrium
permeability progressive water entry, swelling, then
DO NOT DISTRIBUTE © RAT 13
bursting generally directed outward
o Negative charge on impermeant o Net efflux of K and Cl – lowers
intracellular solutes will lead to bursting intracellular solute
- Largely excluding NaCl to counter act – make o Initiated by activating K/Cl
NaCl functionally impermeable cotransporter
o Offsets osmotic effects of intracellular Cells respond to long-term hyperosmolality by
negative charges accumulating new intracellular organic solutes
o Steady state maintained by active Acute response – uptake of salts
transport Long term adaptation – involves accumulation of
- Inside Na, K, Cl are constant because: organic solutes (Osmolytes) within the cell; include:
o Active extrusion of 3 N a for 2 K 1. 2 Impermeant alcohol derivatives of common
o Net flux of Cl is zero sugars (Sorbitol and inositol)
- If Na-K pump inhibited = depolarization 2. 2 amines (betaine and taurine)
o Passive entry of Na ions exceed efflux of Generation of organic solutes (idiogenic osmoles) –
K major role in raising intracellular osmolality; Particularly
o Continuous outward K true in brain cells
o Inside-negative Vm driving force for Sorbitol Glucose catalyzed by ALDOSE REDUCTASE
excluding Cl Cells can also transport them into cytosol from outside
Cl now enters - Use distinct Na-coupled cotransport systems
o Increase in osmotically active particles The gradient in tonicity-or effective osmolality-
o Leads to cell swelling determines the osmotic flow of water across a cell
o Pathways dependent on Na-K pump are membrane
de-energized TBW – distributed in interstitial, intracellular,
Cell volume changes trigger rapid changes in ion transcellular fluids, and blood plasma
channels or transporters, returning volume toward Water Exchange Across Cell Membranes
normal - Increasing hydrostatic pressure in interstitial
Response to Cell Shrinkage space will cause cell to compress – intracellular
- Addition of mannitol – solution becomes hydrostatic increases similarly
Hyperosmolal o H2O does not enter
o Draws H2O out of cells - Increase ECF osmolality – cause H2O to move
- Many types of cells respond by activating solute out of cell
uptake process known as Regulatory Volume - If cell does not have RVI, cell volume will be
Increase (RVI) reduced indefinitely
- Shrinkage activates ubiquitous NHE1 isoform – - Urea penetrates slowly than H2O does
Na-H exchanger o Initial effect is to shrink cell
o Increased uptake of Na, extrusion of H o As it gradually equilibrates across cell
alkalinize cell activates Cl-HCO3 membrane cell reswells to its initial
net effect is entry of NaCl Na-K volume
pump extrude Na FINAL: KCl net gain - Effective osmolality – also known as tonicity
- Resulting increase in intracellular osmoles o Tonicity – includes both Na and glucose
draws water into the cell but not BUN
- RVI response may be mediated by NKCC1 - Isosmolal – 290 mOsm
isoform and the Na/K/Cl cotransporter - Hyperosmolal - >290 mOsm
Response to Swelling - Hypo-somolal - <290 mOsm
- Hypo-osmolal – extracellular osmolality is - Hypertonic, isotonic, hypotonic – comparing one
decreased solution with another solution across well-
o H2O moves in cells defined membrane
- Many cells respond by activating efflux o Isotonic – effective osmolality is same
pathways known as Regulatory Volume with reference solution
Decrease (RVD) o Hypertonic – higher effective osmolality
o Activates Cl or K channels (or both) – o Hypotonic – solution has lower effective
DO NOT DISTRIBUTE © RAT 14
osmolality Effective circulating volume – blood volume necessary
- Shifts of H2O result from alterations in effective to achieve adequate perfusion
ECF osmolality or tonicity Extracellular Na = total-body content
o Changes in tonicity usually caused by Increases in ECF volume OR total-body Na content
decreases in Na in ECF and plasma, renal excretion of Na
increase in Na, or increase in glucose - Plasma Na concentration does not regulate
Water Exchange Across the Capillary Wall renal excretion of Na
- Freely permeable to solutes smaller than Holding Na constant (major part of total body osmoles)
plasma proteins - Increase in TWB would decrease osmolality
- Only net osmotic force that acts by asymmetric o Net gain or loss of solute-free H2O has
distribution of proteins in plasma VS interstitial major impact on osmolality and Na of
fluid the ECF
- Protein osmotic pressure, colloid osmotic o A gain or loss of solute-free H2O affects
pressure, oncotic pressure ICF more than ECF
- Oncotic pressure difference – tends to pull H2O Small decrease in osmolality
from the interstitium - Increase renal water excretion
- Net movements of H2O accompanied by small - Diminished thirst
solutes Emergency states of very low ECF
o Do not contribute significantly to - Try to conserve Na
osmotic driving force - Seek water by triggering thirst
Adding isotonic saline, pure water, or pure NaCl to the - Conserve water by concentrating urine
ECF will increase ECF volume but will have divergent TRANSPORT OF SOLUTES AND WATER ACROSS
effects on ICF volume and ECF osmolality EPITHELIA
Adding solute-free water or NaCl will alter volume and Epithelia – barriers that separate ECF from outside
composition of fluid compartments world
Infusion of Isotonic Saline - Uninterrupted sheet of cells joined by junctional
- NO CHANGE OCCURS complexes
- Efficient way to expand ECF - Serve as selectively permeable barrier
Infusion of “Solute-Free” Water - Demarcate boundary between apical and
- Infusing glucose is equivalent to infusing pure basolateral regions
water because it is metabolized to CO2 and H2O - Capable of VECTORIAL TRANSPORT
- Infusing pure H2O cause cells near the point Apical membrane – brush border, mucosal membrane,
of infusion to burst (UNWISE) or luminal membrane
- Lowers ECF osmolality Basolateral membrane – serosal or peritubular
- Relatively Ineffective means of expanding ECF membrane
o More of added water ended up The epithelial cell generally has different
intracellularly electrochemical gradients across its apical and
- Dilutes osmolality of body fluids basolateral membrane
Ingestion of Pure NaCl Salt - Transepithelial differences occur – composition
- Adding or removing Na will mainly affect ECF of “outside world” not the same as ECF
volume o Transepithelial voltage usually not zero
- Adding or removing solute-free H2O affects - Electrophysiological methods provide 2 major
osmolality of the body types of information:
- Increase osmolality of ECF draws H2O out of o Define electrical driving forces
ICF o Electrical measurements define
- There is increase in water volume in the ECF electrical resistance of epithelium
even though no water was added to the body - Transepithelial voltage – voltage different b/n
- Total-body content of Na is the major solutions on either side of epithelium
determinant of ECF volume - Basolateral cell membrane voltage (Vbl) –
Whole body Na content determines ECF volume, microelectrode into epithelial cell and reference
whereas whole-body content determines osmolality electrode to blood/interstitial space
DO NOT DISTRIBUTE © RAT 15
- Apical cell membrane voltage (Va) – compare across apical membrane provides driving
intracellular electrode with reference electrode force for Na to enter through ENac Na channels
in the lumen in apical membrane
- Va + Vbl = Transepithelial voltage - Na-K pump generates current of positive charge
- From Ohm’s law – possible to calculate the from lumen to interstitium (b/c current is more
electrical resistance of entire wall or positive in interstitium)
transepithelial resistance (Rte) o Creates lumen-negative transepithelial
Tight and Leaky epithelia differ in permeability of their voltage
tight junctions Driving force for passive Cl
- Resistance to flow of electrical current – one absorption through paracellular
measure of how tightly an epithelium separates pathway
o Low electrical resistance – low- K secretion
resistance pathway in tight junctions - K channels at apical membrane allows K from
(leaky) Na-K pump to be secreted across apical
More permeant membrane
o Tight – high electrical resistance o Basis of K secretion
- Transcellular pathway – substance crosses - Amiloride – blocks apical ENaC Na channels
through cell by first passing across apical then inhibits K secretion and Na reabsorption
basolateral membranes Glucose Absorption
- Paracellular pathway – substance passes - Absorbed by secondary active cotransport of Na
through tight junctions and lateral intercellular with organic solutes in SI and proximal tubule
spaces o SGLT for example
- Leaky epithelia – perform bulk transepithelial - Inward Na gradient drives entry of both Na and
transport; not good with maintaining gradients Glucose accumulates inside cell passively
o In proximal tubules and small intestine exits through carrier mediated transporter
- Tight epithelia – maintain transepithelial ion (GLUT)
concentration or osmotic gradients - Can also drive Cl absorption due to lumen-
o Distal nephron, large intestines, and negative transepithelial voltage generated
urinary bladder (tightest) Cl secretion
- Na-K pump is located exclusively at basolateral - Inwardly directed Na drives secondary active Cl
membrane EXCEPT in CHOROID PLEXUS uptake by Na/L/Cl cotransporter NKCC1
- Most of the K recycles back out through K - Cl accumulated can exit through CFTR
channel - Negative charges move across the cell from
o K gradient predominantly determines interstitium to lumen lumen-negative
Vbl voltage drives passive Na secretion across
o Usually 50-60 mV inside negative tight junctions
- Na is lower in epithelial cell than in ECF Water transport across epithelia passively follows
o Active extrusion through Na-K pump solute transport
o Serves as driving force for Na entry H2O moves passively in response to osmotic gradients
through apical Na channels and for Finite permeability of bare lipid bilayer and presence of
secondary active transport of other AQPS ensure rapid equilibriation
solutes Tight junctions provide pathway for H2O
Epithelial cells can absorb or secrete different solutes Hydraulic conductivity – epithelial H2O permeability
by inserting specific channels or transporters at either - Varies widely b/c of:
the apical or basolateral membrane o Differences in membrane lipid
Placing different transporters accomplish net composition
transepithelial transport of different solutes (absorptive o Abundance of AQPs (presence either
or secretory direction) constitutive or regulated)
Na absorption Absorption of Hyperosmotic fluid
- Basolateral Na-K pump pumps Na out - Absorbate is hyperosmotic if epithelium
generates inward Na electrochemical gradient absorbs more salt than water
DO NOT DISTRIBUTE © RAT 16
- In ascending limb of loop of Henle - Passive movement of solutes across tight
o Reabsorbs salt but little water junction can contribute to ‘forward’ or backleak
o Dilute fluid is left behind and renal movement of solute
interstitium is hyperosmotic - Modulate transport by changing permeability of
Absorption of an Isosmotic Fluid pathway
- In renal proximal tubule and small intestine – - Na permeability in proximal tubule increases
no detectable osmotic gradients when ECF volume increases
- Permeability of epithelia for water might be o Lower Na reabsorption b/c of increased
extremely high due to high expression of AQPs backleak of absorbed Na
- Lateral intercellular space – modestly Luminal Supply of Transported Species and Flow Rate
hyperosmotic d/t accumulation of absorbed - Concentration of transported solutes effects
solutes rate of solute transport
o Localized osmotic gradient pull water - Renal proximal tubule – process of glucose
into lateral interspaces absorption depletes glucose slow further
Absorption of Hypo-osmotic fluid absorption
- Active transport of water does not occur o Increase glucose conc’n on site of
- Water cannot move against an osmotic gradeitn glucose uptake increases rate of glucose
- Occurs but requires osmolality of basolateral absorption
membrane exceed apical compartment - Flow itself (sensed by bending central cilium)
- Medullary collecting duct uses this approach trigger signaling cascades resulting in alteration
o Interstitial fluid in renal medulla is of transporter and channel function
hyperosmotic
o H2O permeability is high d/t AQP2
Epithelia can regulate transport by controlling
transport proteins, tight junctions, and the supply of
all transported substance
Increased Synthesis (or Degradation) of Transport
Proteins
- Change number of transport molecules in the
cell
- Aldosterone directly or indirectly increases
transcription rate of genes that encode Na-K
pump
Recruitment of Transport Proteins to the Cell
Membrane
- Store transporters in intracellular organelle
“pool” then inserting them
- Histamine causes “tubulovescles” with H-K
pumps to fuse with apical membrane of gastric
parietal cell
o Initiates gastric secretion
Post-translational Modification of Pre-Existing
Transport Proteins
- cAMP enhance phosphorylation of apical
membrane Cl channel
o CFTR – Cl channel regulated by
phosphorylation
o Defect in regulation of CFTR – primary
physiological abnormality in cystic
fibrosis
Changes in Paracellular Pathway
DO NOT DISTRIBUTE © RAT 17
You might also like
- The Body Fluid Compartments & HomeostasisDocument7 pagesThe Body Fluid Compartments & HomeostasisEstellaNo ratings yet
- Intravenous Fluids:correction Na and KDocument5 pagesIntravenous Fluids:correction Na and KHanako AranillaNo ratings yet
- Fluids and ElectrolytesDocument38 pagesFluids and ElectrolytesJohn Anthony de GùzmanNo ratings yet
- 1 Parcial Ingles CientificoDocument7 pages1 Parcial Ingles Cientificobelemleyva03No ratings yet
- Fluid and Electrolytes - MTDocument14 pagesFluid and Electrolytes - MTAlliah Polvorido AnaudNo ratings yet
- Fluid and ElectrolyteDocument5 pagesFluid and ElectrolytedestiaNo ratings yet
- BLOOD&CARDIOVASCULARDocument29 pagesBLOOD&CARDIOVASCULARGuenNo ratings yet
- 3-s2.0-B9780323532655000150 15 - Disorders of Water Balance Joseph G. VerbalisDocument64 pages3-s2.0-B9780323532655000150 15 - Disorders of Water Balance Joseph G. VerbalisDEWINo ratings yet
- Disorders of Water BalanceDocument64 pagesDisorders of Water BalanceRadley Jed C. PelagioNo ratings yet
- Kati Sriwiyati, Dr. Bagian Fisiologi FK UnswagatiDocument52 pagesKati Sriwiyati, Dr. Bagian Fisiologi FK UnswagatiMelia100% (1)
- Anph Cells and TissuesDocument6 pagesAnph Cells and Tissues屋鈴No ratings yet
- Homeostasis and Fluid ComponentsDocument2 pagesHomeostasis and Fluid ComponentsTanya QuilangNo ratings yet
- BLOODDocument4 pagesBLOODariansofia1031No ratings yet
- Assessment of Fluid and ElectrolyteDocument15 pagesAssessment of Fluid and ElectrolyteDip Ayan MNo ratings yet
- Water and SolutionsDocument16 pagesWater and SolutionsSofia RuanoNo ratings yet
- Acid Base Balance: Dr. Cauan December 2018Document7 pagesAcid Base Balance: Dr. Cauan December 2018Isabel CastilloNo ratings yet
- Surgery I #3 - Fluid and ElectrolytesDocument9 pagesSurgery I #3 - Fluid and ElectrolytesCarl Earvin L. Favorito100% (1)
- PHARMACOLOGYDocument19 pagesPHARMACOLOGYKiela Therese LabroNo ratings yet
- Surgery I Fluids and ElectrolytesDocument14 pagesSurgery I Fluids and Electrolytesrn msnNo ratings yet
- Lecture & Review Guide in HEMATOLOGY 1 & 2 (COMPLETE)Document58 pagesLecture & Review Guide in HEMATOLOGY 1 & 2 (COMPLETE)Kirt Anonuevo100% (3)
- MS ReviewerDocument3 pagesMS Reviewersherlyn galvanNo ratings yet
- Hbioana Le3Document3 pagesHbioana Le3bitangyarahNo ratings yet
- HSSRPTR - 07-Body Fluids and CirculationDocument15 pagesHSSRPTR - 07-Body Fluids and CirculationUnniNo ratings yet
- Intravenous Fluids and Electrolytes: Total Body Fluid CompositionDocument16 pagesIntravenous Fluids and Electrolytes: Total Body Fluid Compositionaulia kamal ansari panggabeanNo ratings yet
- Cell PhysiologyDocument11 pagesCell PhysiologyjandaniellerasNo ratings yet
- Jurnal CairanDocument4 pagesJurnal CairanDokter Daphine SatriaNo ratings yet
- Fluids and ElectrolytesDocument7 pagesFluids and ElectrolytesKarren FernandezNo ratings yet
- Cellular PhysiologyDocument3 pagesCellular PhysiologyMartin AlcantaraNo ratings yet
- 3.14 Chapter 3 Water and Electrolytes Balance and ImblanceDocument140 pages3.14 Chapter 3 Water and Electrolytes Balance and ImblanceShourav SarkarNo ratings yet
- 312 MidDocument60 pages312 MidVALERIANO TRISHANo ratings yet
- Introduction To HematologyDocument2 pagesIntroduction To HematologyJohn Rick OrineNo ratings yet
- Chapter 7Document45 pagesChapter 7fatemaNo ratings yet
- How About Organ Level Regulation?Document55 pagesHow About Organ Level Regulation?Raphael SevillaNo ratings yet
- Physiology of Body FluidsDocument23 pagesPhysiology of Body FluidsRamadan Physiology100% (1)
- Body FluidDocument15 pagesBody FluidrjNo ratings yet
- 3 The Electrochemical Basis of Nerve FunctionDocument33 pages3 The Electrochemical Basis of Nerve FunctionEvets JarusNo ratings yet
- Key Points RevisionDocument124 pagesKey Points RevisionSumeyyah KemalNo ratings yet
- Fluid Balance and Electrolyte Distribution in Human Body BCDocument28 pagesFluid Balance and Electrolyte Distribution in Human Body BCMasoume MohammadiNo ratings yet
- Anhydrase, An Enzyme That Catalyzes TheDocument15 pagesAnhydrase, An Enzyme That Catalyzes TheJohnny eawNo ratings yet
- 1 Biosci The Cells and TissuesDocument7 pages1 Biosci The Cells and TissuesPatricia Rosabelle RoñoNo ratings yet
- Water LoseDocument29 pagesWater LoseJAKLIN EMPOLNo ratings yet
- Chapter 3 - Fluids Electrolytes and Acid Base Therapy - 2012 - Equine SurgeryDocument12 pagesChapter 3 - Fluids Electrolytes and Acid Base Therapy - 2012 - Equine SurgeryVladimir OstriaNo ratings yet
- Mcqs in Medical Physiology 2014 EspDocument138 pagesMcqs in Medical Physiology 2014 EspMusonda MulengaNo ratings yet
- Kes - Cairan TBH ElektrolitDocument31 pagesKes - Cairan TBH ElektrolitDino Syofian TriadiNo ratings yet
- Body Fluids 1 and 2: ObejctivesDocument15 pagesBody Fluids 1 and 2: ObejctivesJoanne Bernadette AguilarNo ratings yet
- 1 Hema LecDocument5 pages1 Hema LecAngela ReyesNo ratings yet
- 1 Hema LecDocument5 pages1 Hema LecAngela ReyesNo ratings yet
- Blood (Notes)Document12 pagesBlood (Notes)Angel Rose BrillanteNo ratings yet
- Chemical Composition of Blood Cells: MLS 302: Basic HaematologyDocument13 pagesChemical Composition of Blood Cells: MLS 302: Basic HaematologyBarakat IsmailNo ratings yet
- Reviewer Chapter 52 FluidsDocument8 pagesReviewer Chapter 52 FluidsKeren GaciasNo ratings yet
- Fluid and ElectrolyteDocument19 pagesFluid and ElectrolyteahmedNo ratings yet
- CELLMOLDocument3 pagesCELLMOLJhon Rey LagosNo ratings yet
- Chapter Blood: RBC Platelet HemostasisDocument89 pagesChapter Blood: RBC Platelet Hemostasisapi-19916399100% (1)
- RBC Membrane DeformabilityDocument16 pagesRBC Membrane DeformabilityMyedelle SeacorNo ratings yet
- Blood ReviewerDocument7 pagesBlood ReviewerKate Dela cruzNo ratings yet
- Blood Chemistry - Dra. Gabaldon (RECT) PDFDocument7 pagesBlood Chemistry - Dra. Gabaldon (RECT) PDFRachel AsuncionNo ratings yet
- Body Fluid, MML, 2021Document51 pagesBody Fluid, MML, 2021Boon AimanNo ratings yet
- Cellular Level of OrganizationDocument9 pagesCellular Level of OrganizationAbby Claire SomeraNo ratings yet
- BloodDocument6 pagesBloodAlther LorenNo ratings yet
- Human Chromosomes: An Illustrated Introduction to Human CytogeneticsFrom EverandHuman Chromosomes: An Illustrated Introduction to Human CytogeneticsRating: 5 out of 5 stars5/5 (1)
- Attended Pta General AssembliesDocument1 pageAttended Pta General AssembliesArlie BalucaNo ratings yet
- Fruit Salad Is A General Term Referring To A Fruit DishDocument1 pageFruit Salad Is A General Term Referring To A Fruit DishArlie BalucaNo ratings yet
- Steps in Registration of PropertyDocument5 pagesSteps in Registration of PropertyLiezel NibresNo ratings yet
- Ao 2 s09 Rules and Procedures Governing The Acquisition and Distribution PDFDocument96 pagesAo 2 s09 Rules and Procedures Governing The Acquisition and Distribution PDFKhryz CallëjaNo ratings yet
- Ethylbenzene DehydrogenationDocument12 pagesEthylbenzene DehydrogenationCeciNo ratings yet
- Form 3 Chemistry Assignment (WK 8)Document3 pagesForm 3 Chemistry Assignment (WK 8)JOSEPH MWANGINo ratings yet
- Russell J. Donnelly - Fifty-Five Years of Taylor - Couette FlowDocument35 pagesRussell J. Donnelly - Fifty-Five Years of Taylor - Couette FlowQMDhidnwNo ratings yet
- Ce6411 Strength of Materials Laboratory (Civil)Document32 pagesCe6411 Strength of Materials Laboratory (Civil)KishanKanhaiyaNo ratings yet
- Aceite de Coco Producido en Nicaragua.Document1 pageAceite de Coco Producido en Nicaragua.Juan Herrera SalazarNo ratings yet
- A Coupled Thermal-Granular Model in Flights Rotary Kiln: Industrial Validation and Process DesignDocument12 pagesA Coupled Thermal-Granular Model in Flights Rotary Kiln: Industrial Validation and Process DesignDouglas TondelloNo ratings yet
- Pilot Product Catalogue 18Document28 pagesPilot Product Catalogue 18veerraju tvNo ratings yet
- Advanced Engineering, Worldwide Facilities & Comprehensive Technical SupportDocument12 pagesAdvanced Engineering, Worldwide Facilities & Comprehensive Technical SupportCenon MalabananNo ratings yet
- Shield Water Heater LiteratureDocument4 pagesShield Water Heater LiteratureJohn MoreNo ratings yet
- Lesson Plan in Cleaning and Sanitizing, Materials FinalDocument6 pagesLesson Plan in Cleaning and Sanitizing, Materials FinalJ'LvenneRoz AnepolNo ratings yet
- Bending Moment in A BeamDocument8 pagesBending Moment in A BeamLim Ksoon100% (1)
- Appendix 7.: Nutritional Goals For Age-Sex Groups Based On Dietary Reference Intakes &Document2 pagesAppendix 7.: Nutritional Goals For Age-Sex Groups Based On Dietary Reference Intakes &GloryJaneNo ratings yet
- TOPIC 1 Stoichiometric Relationships Part 3Document22 pagesTOPIC 1 Stoichiometric Relationships Part 3Kylie ChuaNo ratings yet
- DARPA GaN AdvancesDocument4 pagesDARPA GaN Advancesbring it onNo ratings yet
- E Clips CurvesDocument78 pagesE Clips Curvesner68No ratings yet
- Potassium Nitrate - Refined Grade - Thermosolar - Crystals - June14Document1 pagePotassium Nitrate - Refined Grade - Thermosolar - Crystals - June14JOSE LUISNo ratings yet
- Blasting and PaintingDocument64 pagesBlasting and PaintingSyahril Aizal Ahmad75% (4)
- Final Exam Review 1528317655806 SCDocument18 pagesFinal Exam Review 1528317655806 SCAdil KhurshaidNo ratings yet
- Permeability Plugging Apparatus Instruction ManualDocument60 pagesPermeability Plugging Apparatus Instruction ManualHamed NazariNo ratings yet
- Soil Quality - Sampling - General Requirements: Vietnam Standard TCVN 5297: 1995Document3 pagesSoil Quality - Sampling - General Requirements: Vietnam Standard TCVN 5297: 1995huytai8613No ratings yet
- Basic Nuclear Physics: Day 1-Lecture 1Document35 pagesBasic Nuclear Physics: Day 1-Lecture 1jody9090No ratings yet
- Service ProductsDocument122 pagesService ProductsIswahyudi Aprilyastono100% (1)
- GRCDocument3 pagesGRCAristo OnanNo ratings yet
- 1 - A History of Chemical EngineeringDocument58 pages1 - A History of Chemical Engineeringbunchuu100% (1)
- 12th Chemistry Cbse Board Paper 2008 To 12 (Solved)Document285 pages12th Chemistry Cbse Board Paper 2008 To 12 (Solved)jaisabhi01570% (27)
- Oils and Fats BrochureDocument12 pagesOils and Fats BrochurerodrigoolmedoNo ratings yet
- Anthropogenic Activities and Water Quality in Estero de Binondo, ManilaDocument92 pagesAnthropogenic Activities and Water Quality in Estero de Binondo, ManilaClayd Genesis CapadaNo ratings yet
- ISO 6784 82 Concrete-Determination of Static Modulus of Elasticity in CompressionDocument8 pagesISO 6784 82 Concrete-Determination of Static Modulus of Elasticity in CompressionKaan TekinturhanNo ratings yet
- Soot & ScaleDocument12 pagesSoot & ScaleLow Shen WeiNo ratings yet
- Fundamental EquationDocument7 pagesFundamental Equationlmcristina5No ratings yet