Professional Documents
Culture Documents
Metallurgy Notes
Uploaded by
Ellen GraceCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Metallurgy Notes
Uploaded by
Ellen GraceCopyright:
Available Formats
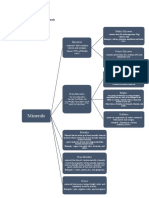
Metallurgy gangue: phosphates,
chlorites, hematite,
The science and art of extracting metals from their ores, refining them, and preparing them for use magnetite
Types:
Physical metallurgy Factors which control the suitability and value of mineral deposits as sources of metal
Extractive metallurgy 1. accessibility of the deposit and proximity to fuel, power and water supplies, etc.
Adaptive metallurgy 2. The form and concentration of the metal (ex Ni ores: laterite has 0.3% Ni, limonite has 2% Ni)
Physical metallurgy 3. The demand for, and value of, the metal
The science and technology of metals and alloys and the effects of processing, composition and 4. The nature of the gangue
environment on their properties
5. The aggregation (size) and dissemination (distribution) of the valuable mineral
The desired properties may be electrical, mechanical, magnetic, or chemical in nature; all of them can be
enhanced by alloying and heat treatment 6. The nature and concentration of metallic impurities or secondary values
Extractive metallurgy Aggregation and dissemination refers to size and distribution
The practice of removing valuable metals from an ore and refining the extracted raw metals into
Minimum
purer metal content:
form
Non-ferrous ores = < 1% metal
Adaptive metallurgy
Gold may be recovered 6ppm of the metal
Casting
Low grade iron ores = <20% metal
Forming
Crystals of cassiterite, a commercially valuable mineral, in a matrix of quartz, the gangue
Machining
Classification of ores
Joining of metals
1.Native ores – the metal is present in the elementary form
Occurrence of metals
2.Sulphide ores – contain the metal in the form of a sulphide
1. Metals in the crust of the earth
3.Oxidised ores – the valuable mineral may be present as oxide, sulphate, silicate, carbonate, or some
2. sea-bed deposits hydrated form of these
-gold and platinum are found principally in the native or metallic form 4.Complex ores – contain profitable amounts of more than one valuable mineral
-Silver, copper and mercury are found native in the form of sulphides (with sulphur), carbonates
Classification
(CO2-3) of ores according to their gangues
and chlorides (Cl-)
-Calcareous or basic (lime rich)
More reactive metals
-Siliceous or acidic (silica rich)
-Oxides and sulphides
Mineral processing
(ex. Of iron)
-Also known as ore dressing, mineral dressing or milling, follows mining and prepares the ores for
-Oxides and silicates extraction of the valuable metal
(ex. Of aluminum and beryllium) -a process of physically separating the grains of valuable minerals from the gangue minerals to produce an
enriched portion, or concentrate (containing most of the valuable minerals) and a discard, or tailing
Silicates – silicon bearing anions (containing the gangue minerals)
Minerals CONCENTRATION
(enrichment process)
-Naturally occurring compounds (were given names according to their composition
-Pyrometallurgical process (extraction and refining of metals at higher temperatures)
-Ex: Galena (PbS) – lead sulphide
-Hydrometallurgical process (The treatment of metal or the separation of metal from ores and ore
Sphalerite (ZnS) – zinc sulphide concentrates by liquid processes)
cassiterite (SnO2) – tin oxide FUNDAMENTAL METHODS OF MINERAL PROCESSING
• Eight elements account for over 99% of the earth’s crust -Liberation-Liberation is accomplished by comminution
- 74.6% (Si and O) (the release of the valuable minerals from their waste gangue minerals)
- 2% (Al, Fe, and Mg) -Separation of the valuables from the gangue
- <0.1% other useful metals (ex: Cu – 0.0055%) (concentration)
ORE physical methods of separation
-Mixtures of extractable minerals and extraneous rocky material 1. Separation dependent on optical properties, etc. (ex: sorting)
Can be described briefly as an accumulation of mineral in sufficient quantity as to be capable 2.
of Separation
economic dependent on specific gravity differences (mass effects)
extraction
3. Separation utilizing the different surface properties of the minerals (froth flotation)
Galena (PbS) – Lead ore
4. Separation dependent on magnetic properties
Chalcopyrite (CuFeS2) – Copper ore
5.Separation dependent on electrical conductivity properties (high-tension, electrostatic separation)
Cinnabar (HgS) – mercury ore (mercuric sulfide)
FLOWSHEET
Galena valuable: Pb
- shows the sequence of operations in the plant
gangue: Cu, Zn, quartz,
calcite - Can be presented as a block diagram in which all operations of one character are
grouped
Chalcopyrite valuables: Ag, Cu
(+) – oversized material returned for further treatment
(-) – undersized material allowed to proceed to the next stage
ORE HANDLING
-Which may account for 30-60% of the total delivered price of raw materials, covers the
process of transportation, storage, feeding and washing of the ore en route to, or during,
its various stages of treatment in the mill
Removal of harmful materials
-Iron and steel can jam the machinery
-Wood can be ground into fine pulp and causes choking or blocking of screens, choking
flotation cell ports, consume flotation reagents by adsorption and decompose to give
depressants, which hinder valuable minerals unfloatable
-Clays and slimes will hinder screening, filtration and thickening , and consume reagents
Sampling and weighing of ore
Sampling is the means whereby a small amount of material is taken from main bulk in
such a manner that it is representative of that larger amount
Weighing and sampling should be carried out before the material is subject to losses in
the mill
Take at least 5% of the total weight if the ore as a primary sample
Moisture sampling
-Moisture samples and assay samples must be taken from a point near to the weighing
equipment
-Grab sampling is commonly used
- Least accurate but the cheapest and the most rapid
- Samples must be collected and placed in a sealed container
% moisture =
Samples should not be dried at temperatures above 105oC
Assay sampling
-The bulk sample requires thorough drying and mixing before further division to produce
a reasonable size for assay
-The bulk sample should be made as homogeneous as possible
-If complete homogeneity is achieved, every increment obtained by the sampling
method will be representative of the material
-Coning and quartering
-The oldest method
-It is used in dividing small quantities of material
-Consists of pouring the material into a conical heap and relying on its radial symmetry
to five 4 identical samples when the heap is flattened and divided by a cross-shaped
metal cutter
-2 opposite corners are taken as the sample, the other corners are discarded
-The portion chosen as the sample may again be coned and quartered
The process continued until a sample of the required size is produced
The Jones riffle
-This splitter is an open V-shaped box in which a series of chutes is mounted at right
angles to the long axis
-Sample is poured into the chute and split into equal portions by the slots, until after
repeated cycles a sample of the desired size is obtained
You might also like
- Earthy Materials and ProcessesDocument5 pagesEarthy Materials and ProcessesAngèll RòseNo ratings yet
- Chapter2 Lesson2.4 Ores and MineralsDocument4 pagesChapter2 Lesson2.4 Ores and MineralsMeiko DesantoresNo ratings yet
- Test Scribd DocumentDocument30 pagesTest Scribd DocumentShudhanshu KumarNo ratings yet
- Metallurgy Extraction ProcessDocument16 pagesMetallurgy Extraction ProcessT. NikhilNo ratings yet
- Tech 8 6 PDFDocument85 pagesTech 8 6 PDFNaths BarreraNo ratings yet
- Chemistry of Heavier Elements Metallurgy GuideDocument34 pagesChemistry of Heavier Elements Metallurgy GuideNatish JaglanNo ratings yet
- Extraction of MetalsDocument31 pagesExtraction of MetalsTadiwa RylyNo ratings yet
- Mineral Processing Lecture NotesDocument943 pagesMineral Processing Lecture NotesGeorge GomezNo ratings yet
- Occurrence and Extraction of Metals: Module - 6Document15 pagesOccurrence and Extraction of Metals: Module - 6Manish kumarNo ratings yet
- Introduction To Mineral Processing Lecture-1Document52 pagesIntroduction To Mineral Processing Lecture-1Sammy SinghaniaNo ratings yet
- Mineral DepositsDocument2 pagesMineral DepositsFrancesca YanzonNo ratings yet
- 1 Ore Deposits: Iron Ore (Banded Iron Formation)Document5 pages1 Ore Deposits: Iron Ore (Banded Iron Formation)David SilverNo ratings yet
- Bahan Galian Logam - PPTDocument26 pagesBahan Galian Logam - PPTDelia AhmadNo ratings yet
- Economic Geology: Introduction to Earth's Valuable Mineral ResourcesDocument16 pagesEconomic Geology: Introduction to Earth's Valuable Mineral ResourcesRananjay SinghNo ratings yet
- 1 - Kuliah (1) - Pengantar PertambanganDocument26 pages1 - Kuliah (1) - Pengantar PertambanganGabriela Elisabeth TasidjawaNo ratings yet
- METALLURGYDocument4 pagesMETALLURGYdevanshramchandani11No ratings yet
- 313 E Book2 PDFDocument453 pages313 E Book2 PDFdanhemNo ratings yet
- Occurrence and Extraction of Metals: Module - 6Document20 pagesOccurrence and Extraction of Metals: Module - 6TeachingTrainingCoaching KnowledgeSharingSessionNo ratings yet
- Classifying Minerals by Composition and PropertiesDocument1 pageClassifying Minerals by Composition and PropertiesBeommie ChoiNo ratings yet
- Minerals & Rocks: Are They Important?Document33 pagesMinerals & Rocks: Are They Important?sophia gloriosoNo ratings yet
- Met Ta LurgyDocument35 pagesMet Ta Lurgyakshay1203No ratings yet
- MetalsDocument2 pagesMetalssong MusicNo ratings yet
- 3 Minerals PDFDocument3 pages3 Minerals PDFJasmine Bianca CastilloNo ratings yet
- Mining GeologyDocument40 pagesMining Geologyyannis magan80% (5)
- Metallurgy Theory PDFDocument13 pagesMetallurgy Theory PDFUtkarsh RaiNo ratings yet
- Introduction To Mineral Processing 2010Document24 pagesIntroduction To Mineral Processing 2010elmonemNo ratings yet
- Isolation of ElementsDocument34 pagesIsolation of ElementsSai Sasivardhan GampaNo ratings yet
- Gold Process Mineralogy and Its Significance in Gold MetallurgyDocument7 pagesGold Process Mineralogy and Its Significance in Gold MetallurgyStefania HernandezNo ratings yet
- O Level Geography Chap Mineral Resources Full NotesDocument23 pagesO Level Geography Chap Mineral Resources Full NotesAmna Faisal73% (15)
- MetalluricasDocument27 pagesMetalluricasSupreeth PremkumarNo ratings yet
- Lecture Notes - General Principles and Isolation of ElementsDocument25 pagesLecture Notes - General Principles and Isolation of ElementsYohance NayakNo ratings yet
- Metals and Metallurgy FundamentalsDocument26 pagesMetals and Metallurgy FundamentalsParas Chand ThaqureeNo ratings yet
- Ore Is Natural Rock or Sediment That Contains Desirable Minerals, TypicallyDocument5 pagesOre Is Natural Rock or Sediment That Contains Desirable Minerals, TypicallyFlaviaBritesNo ratings yet
- Definition of Ore Types and MineralsDocument2 pagesDefinition of Ore Types and Mineralssubanandam567No ratings yet
- 2000 Mineral Deposits Vol 79 Suppl1!14!22Document9 pages2000 Mineral Deposits Vol 79 Suppl1!14!22Cristi CrisNo ratings yet
- NotesDocument24 pagesNotesIsrael SapnuNo ratings yet
- Topic 6 - Minerals and Its PropertiesDocument1 pageTopic 6 - Minerals and Its Propertieslesly AnneNo ratings yet
- Minerals - RocksDocument67 pagesMinerals - RocksChandramala ANo ratings yet
- Iron Ore - Ground Breaking PerspectiveDocument15 pagesIron Ore - Ground Breaking PerspectivecoderNo ratings yet
- 1 Lecture Metallogenic Zones in PakistanDocument74 pages1 Lecture Metallogenic Zones in PakistanFawad AhmedNo ratings yet
- 1 Lecture Metallogenic Zones in PakistanDocument74 pages1 Lecture Metallogenic Zones in PakistanFawad AhmedNo ratings yet
- WK 3 EARTHDocument23 pagesWK 3 EARTHClaire ChanNo ratings yet
- Mineral Deposit: MetalDocument22 pagesMineral Deposit: Metal2fercepolNo ratings yet
- METALLURGY GUIDEDocument2 pagesMETALLURGY GUIDEuniquestarNo ratings yet
- 1-Endapan Mineral - PendahuluanDocument22 pages1-Endapan Mineral - Pendahuluanwahyu1507.0903No ratings yet
- Metals and Metallurgical PrinciplesDocument13 pagesMetals and Metallurgical PrinciplesManoj KhanalNo ratings yet
- Minerals and Rocks Lesson for Grade 11 StudentsDocument7 pagesMinerals and Rocks Lesson for Grade 11 StudentsSophia CabalhaoNo ratings yet
- G8 Q4 Rocks and MineralsDocument49 pagesG8 Q4 Rocks and MineralsLocal ClownNo ratings yet
- Gold Extraction and Recovery Processes: Wong Wai Leong Eugene and Arun S. MujumdarDocument20 pagesGold Extraction and Recovery Processes: Wong Wai Leong Eugene and Arun S. MujumdarAmsalNo ratings yet
- General Principles and Process of Isolating ElementsDocument32 pagesGeneral Principles and Process of Isolating ElementsGayatri GNo ratings yet
- 5 - 18b - Mineral ResourcesDocument90 pages5 - 18b - Mineral ResourcesDa Apollyon100% (4)
- Principles of Extractive Metallurgy (MT31015) : Metal Occurrence and Extraction ProcessDocument57 pagesPrinciples of Extractive Metallurgy (MT31015) : Metal Occurrence and Extraction Processtrishaa balajiNo ratings yet
- Minerals Classification GuideDocument11 pagesMinerals Classification Guidesubham kunduNo ratings yet
- Basic Principle of Extraction of IronDocument35 pagesBasic Principle of Extraction of IronHimanshuNo ratings yet
- DPP 02 Chemical Bonding JH Sir-4165Document28 pagesDPP 02 Chemical Bonding JH Sir-4165Prabhakar BandaruNo ratings yet
- Module 2 Rocks and MineralsDocument58 pagesModule 2 Rocks and MineralsRhianne Ahmithy Del RosarioNo ratings yet
- OreDocument5 pagesOreYeyint AungNo ratings yet
- Geologic Resource Formation FactorsDocument29 pagesGeologic Resource Formation FactorsJose Mari DichosonNo ratings yet
- Little Rocks & Small Minerals! | Rocks And Mineral Books for Kids | Children's Rocks & Minerals BooksFrom EverandLittle Rocks & Small Minerals! | Rocks And Mineral Books for Kids | Children's Rocks & Minerals BooksRating: 4 out of 5 stars4/5 (1)
- 2009 RI Prelims Chem H2 P1 QPDocument16 pages2009 RI Prelims Chem H2 P1 QPniveumaNo ratings yet
- Matter Packet PDFDocument6 pagesMatter Packet PDFNopporn SaSaNo ratings yet
- PT in Music GroupingsDocument1 pagePT in Music GroupingsGabriel BantayNo ratings yet
- 181 PDFDocument2 pages181 PDFPijush SarkarNo ratings yet
- Gases and Solutions QPDocument13 pagesGases and Solutions QPGbenga AjibikeNo ratings yet
- Catalogus 2014 Compleet - LR PDFDocument215 pagesCatalogus 2014 Compleet - LR PDFJacob ReedNo ratings yet
- Filling Order 2Document52 pagesFilling Order 2Kram M Razatlab100% (1)
- 0654 w18 QP 21Document16 pages0654 w18 QP 21lddangNo ratings yet
- PPG73APR11EXPDocument469 pagesPPG73APR11EXPAmritNo ratings yet
- Zinc Chrome-Py 36 TdsDocument1 pageZinc Chrome-Py 36 TdsIliass MeskiNo ratings yet
- Reactivity Series WorksheetDocument6 pagesReactivity Series WorksheetjanithaNo ratings yet
- Cambridge International General Certificate of Secondary EducationDocument16 pagesCambridge International General Certificate of Secondary EducationkdebipershadNo ratings yet
- Soalan Latihan Tambahan TermokimiaDocument5 pagesSoalan Latihan Tambahan TermokimiaZulNo ratings yet
- Piping Codes & Standard and Cross ReferrenceDocument12 pagesPiping Codes & Standard and Cross ReferrencesmaluqNo ratings yet
- POC - 1 & Structural Identification Theory - EDocument10 pagesPOC - 1 & Structural Identification Theory - EthinkiitNo ratings yet
- Sodium Hydroxide Production With A Calcium CarbonaDocument7 pagesSodium Hydroxide Production With A Calcium CarbonaFebri SandiNo ratings yet
- Brochure Covalumine PigmentsDocument3 pagesBrochure Covalumine PigmentsMamadou Lamine FallNo ratings yet
- Atomic Structure and Properties of HydrogenDocument77 pagesAtomic Structure and Properties of HydrogenRohith Kumar100% (1)
- Paper 3 Nov 2008Document8 pagesPaper 3 Nov 2008MSHNo ratings yet
- Ref Manual - Additives For MA Spinel PDFDocument5 pagesRef Manual - Additives For MA Spinel PDFRITWIK SARKARNo ratings yet
- Elements Compounds and MixturesDocument28 pagesElements Compounds and MixturesDionisio BrinosaNo ratings yet
- Final Review PDFDocument10 pagesFinal Review PDFKatheeja MusatheekNo ratings yet
- Properties BookDocument19 pagesProperties BookEarl CopeNo ratings yet
- OCR Chemistry Exam Question Booklet 2Document37 pagesOCR Chemistry Exam Question Booklet 2krnc_11No ratings yet
- Electrolytic Zinc Coatings (Extract From Iso 4042)Document2 pagesElectrolytic Zinc Coatings (Extract From Iso 4042)Abhishek DhawanNo ratings yet
- Absorbable Dusting PowderDocument1 pageAbsorbable Dusting PowderRaquel BcNo ratings yet
- Dwnload Full C Programming From Problem Analysis To Program Design 8th Edition Malik Solutions Manual PDFDocument36 pagesDwnload Full C Programming From Problem Analysis To Program Design 8th Edition Malik Solutions Manual PDFlucedystu56100% (13)
- Aqa 8464C1F QP Jun18 PDFDocument24 pagesAqa 8464C1F QP Jun18 PDFMustafa NabeihNo ratings yet
- Maharashtra-HSC-d & F Paper-2 TargetDocument39 pagesMaharashtra-HSC-d & F Paper-2 TargetkrritikksNo ratings yet