Professional Documents
Culture Documents
Flecainide
Uploaded by
Alexandra Antondy0 ratings0% found this document useful (0 votes)
376 views3 pagesOriginal Title
flecainide.docx
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
376 views3 pagesFlecainide
Uploaded by
Alexandra AntondyCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 3
Mindanao State University – Iligan Institute of Technology Student: Bianca Mikaela F.
Dosdos
Block: 262
PHARMACOLOGY
DRUG STUDY
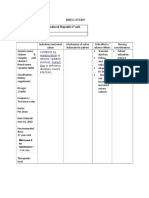
Brand Name: Tambocor Generic Name: Flecainide Drug Classification: Anti-arrhythmics
Dosage, Route & Frequency
Drug-Drug & Drug-Food Side Effects Adverse Reactions (By
Recommende Drug Action Indications Contraindications
Prescribed Interactions (By System) System)
d
Ventricular Slows Drug-Drug: Life-threatening Hypersensitiv CNS: CNS:
Tachycardia conduction in qrisk of arrhythmias with ventricular ity dizziness, anxiety, dizziness, anxiety,
PO (Adults): cardiac tissue other antiarrhythmics, arrhythmias, Cardiogenic fatigue, headache, fatigue, headache,
100 mg q 12 hr by altering including calcium channel including shock mental depression. mental depression.
initially,qby 50 transport of blockers. Disopyramide, ventricular EENT: EENT:
mg twice daily ions across cell beta blockers, tachycardia. blurred vision, visual blurred vision, visual
until response membranes. orverapamilmay have Supraventricular disturbances. disturbances.
is obtained or qmyocardial depressant tachyarrhythmias CV: CV:
maximum effects; combination use including: ARRHYTHMIAS, CHEST ARRHYTHMIAS, CHEST
total daily should be undertaken Paroxysmal PAIN, HF. GI:anorexia, PAIN, HF. GI:anorexia,
dose of 400 cautiously. Amiodarone supraventricular constipation, drug- constipation, drug-
mg is reached. doubles serum flecainide tachycardia induced hepatitis, induced hepatitis,
Some patients levels (pflecainide dose by (PSVT), nausea, stomach pain, nausea, stomach pain,
may require q 50%).qserum digoxin levels Paroxysmal atrial vomiting. vomiting.
8 hr dosing. by a small amount (15– fibrillation/flutter Derm: Derm:
Renal 25%). Concurrent beta (PAF). Unlabeled rash. rash.
Impairment blocker therapy may Use: Single dose Neuro: Neuro:
PO (Adults): causeqlevels of beta treatment of tremor. tremor.
CCr 35 blocker and flecainide. atrial fibrillation.
mL/min—100 Alkalinizing agents
mg once a day promote
or 50 mg q 12 reabsorption,qblood levels,
hr initially; and may cause toxicity.
further dosing Acidifying agentsqrenal
on the basis of elimination and
frequent blood maypeffectiveness of
level flecainide (if urine pH 5).
monitoring.
PSVT/PAF PO
(Adults): 50 Drug-Food:
mg q 12 hr Foods thatqurine pH to
initially,qby 50
mg twice daily 7 result inqlevels (strict
until response vegetarian diet). Foods or
is obtained or beverages thatpurine pH to
maximum 5qrenal elimination and
total daily mayp effectiveness of
dose of 300 flecainide(acidic juices)
mg is reached.
Some patients
may require q
8 hr dosing.
Atrial
Fibrillation
(unlabeled) PO
(Adults): 200
mg or 300 mg
single dose.
Responsibilities in the Nursing Process (ADPIE) Responsibilities in the Nursing Process (ADPIE)
Assessment Patient/Family Teaching
● Monitor ECG or Holter monitor prior to and periodically duringtherapy. May cause ● Instruct patient to take medication around the clock as directed at evenly spaced intervals, even if
QRS widening, PR prolongation, and QT prolongation. feeling better. Take missed doses as soon as remembered if within 6 hr; omit if remembered later.
● Monitor BP and pulse periodically during therapy. Gradual dose reduction may be necessary.
● Monitor intake and output ratios and daily weight. Assess patient for signs of HF ● May cause dizziness or visual disturbances. Caution patient to avoid driving and other activities
(peripheral edema, rales/crackles, dyspnea, weight gain, jugular venous distention). requiring alertness until response to medication is known.
● Lab Test Considerations: Evaluate renal, pulmonary, and hepatic functions and CBC ● Advise patient to notify health care professional of medication regimen prior to treatment or surgery.
periodically on patients receiving long-term therapy. Flecainide should be discontinued ● Instruct patient to notify health care professional if chest pain, shortness of breath, or diaphoresis
if bone marrow depression occurs. occurs.
● May causeqin serum alkaline phosphatase during prolonged therapy. ● Advise patient to carry identification describing disease process and medication regimen at all times.
● Toxicity and Overdose: Therapeutic blood levels range from 0.2 to 1.0 mcg/ mL. Evaluation/Desired Outcomes
Monitor plasma trough levels frequently during dose adjustment in patients with ● Decrease in frequency of life-threatening ventricular arrhythmias.
severe renal or hepatic disease or in patients with HF and moderate renal ● Decrease in supraventricular tachyarrhythmias.
impairment.Potential Nursing Diagnoses
Decreased cardiac output (Adverse Reactions)
Implementation
● Do not confuse Tambocor with Pamelor.
● Previous antiarrhythmic therapy (except lidocaine) should be withdrawn 2– 4 half-
lives before starting flecainide.
● Therapy should be initiated in a hospital setting to monitor for increase in
arrhythmias.
● Dose adjustments should be at least 4 days apart because of the long half-life of
flecainide.
Sources:
https://davisplus.fadavis.com/3976/meddeck/pdf/flecainide.pdf
https://www.webmd.com/drugs/2/drug-6109/flecainide-oral/details
https://reference.medscape.com/drug/tambocor-flecainide-342300
You might also like
- Activase for Acute Ischemic Stroke and Heart ConditionsDocument3 pagesActivase for Acute Ischemic Stroke and Heart Conditionsmharjoe pulmanoNo ratings yet
- Mindanao State University - Iligan Institute of Technology Student: ALEXA MURIEL L. MOZAR Section: BLOCK 261Document2 pagesMindanao State University - Iligan Institute of Technology Student: ALEXA MURIEL L. MOZAR Section: BLOCK 261Alexandra AntondyNo ratings yet
- Nifedipine and Prednisone Drug StudyDocument5 pagesNifedipine and Prednisone Drug StudyAllyne GavinoNo ratings yet
- Mupirocin Drug StudyDocument1 pageMupirocin Drug StudyArthur Christopher Corpuz0% (1)
- DRUG-STUDY - BALLON, Karlo CDocument6 pagesDRUG-STUDY - BALLON, Karlo CMelinda Cariño BallonNo ratings yet
- NCP & Drug Study (Tondo Med)Document5 pagesNCP & Drug Study (Tondo Med)Kevin_Remollo_2431No ratings yet
- Vit K Drug StudyDocument2 pagesVit K Drug StudyKrisha AristonNo ratings yet
- Ketorolac: Uses, Dosing, Side EffectsDocument14 pagesKetorolac: Uses, Dosing, Side EffectsVin LandichoNo ratings yet
- Drug StudyDocument21 pagesDrug StudyShyla Garnace JavillonarNo ratings yet
- DrugStudy - CamaristaColeenMaeC (BSN III-G) (Prednisone)Document2 pagesDrugStudy - CamaristaColeenMaeC (BSN III-G) (Prednisone)Coleen Mae CamaristaNo ratings yet
- Drug Study FinalDocument5 pagesDrug Study FinalJackie Ann Marie DapatNo ratings yet
- Drug Study - OB WardDocument8 pagesDrug Study - OB WardCheska YsabelleNo ratings yet
- Drug Study SummaryDocument7 pagesDrug Study SummaryKateLayaogNo ratings yet
- Ertapenem (Invanz)Document1 pageErtapenem (Invanz)Adrianne BazoNo ratings yet
- Amoxicillin Nursing ConsiderationsDocument3 pagesAmoxicillin Nursing ConsiderationsNico DonatoNo ratings yet
- Vii. Drug Study Drug Mechanism of ActionDocument7 pagesVii. Drug Study Drug Mechanism of ActionRifa'atul MahmudahNo ratings yet
- Drug Mechanis MOF Action Indicatio N Contraindicatio N Side Effects Adverse Effects Nursing Responsibilit YDocument1 pageDrug Mechanis MOF Action Indicatio N Contraindicatio N Side Effects Adverse Effects Nursing Responsibilit YNica RodriguezNo ratings yet
- LacipilDocument2 pagesLacipilianecunarNo ratings yet
- Drug Study FDocument3 pagesDrug Study FFatima Love Ariate-ArcasetasNo ratings yet
- Drug StudyDocument24 pagesDrug StudyMc Joewell HudencialNo ratings yet
- Drug Study Cushing's SyndromeDocument5 pagesDrug Study Cushing's SyndromeSelena MarieNo ratings yet
- Sal But AmolDocument2 pagesSal But AmolKay MirandaNo ratings yet
- Drug StudyDocument13 pagesDrug StudyAldrin Ian Oraza AlpeNo ratings yet
- Azithromycin, Cefixime, Paracetamol Drug StudyDocument4 pagesAzithromycin, Cefixime, Paracetamol Drug StudyAzizah VillaminNo ratings yet
- Azithromycin Dosage Indications Adverse Effects NursingDocument1 pageAzithromycin Dosage Indications Adverse Effects NursingGrape JuiceNo ratings yet
- PHINMA - UNIVERSITY OF ILOILO DRUG STUDY ON ROPIVACAINE/BUPIVACAINEDocument2 pagesPHINMA - UNIVERSITY OF ILOILO DRUG STUDY ON ROPIVACAINE/BUPIVACAINErica sebabillonesNo ratings yet
- Dutasteride 0.5mg + Tamsulosin HCL 0.4mg (Duodart)Document19 pagesDutasteride 0.5mg + Tamsulosin HCL 0.4mg (Duodart)ddandan_2No ratings yet
- Pathophysiology of Nephrotic SyndromeDocument1 pagePathophysiology of Nephrotic SyndromeKristian Karl Bautista Kiw-isNo ratings yet
- Phenylephrine HydrochlorideDocument5 pagesPhenylephrine HydrochlorideRoger Jr PumarenNo ratings yet
- Cefoxitin Drug StudyDocument1 pageCefoxitin Drug StudyArthur Christopher CorpuzNo ratings yet
- Imdur for Angina Relief: Brand and Generic Names, Uses, Dosage, Side EffectsDocument2 pagesImdur for Angina Relief: Brand and Generic Names, Uses, Dosage, Side Effectslalyn_lumagbasNo ratings yet
- Name of Drug SoludexideDocument2 pagesName of Drug SoludexideSian AsadaNo ratings yet
- Drug Study PsychiaDocument10 pagesDrug Study PsychiaIRA MONIQUE CABADENNo ratings yet
- CefuroximeDocument11 pagesCefuroximeAlmira Ballesteros CestonaNo ratings yet
- Drug StudyDocument7 pagesDrug StudyHerwincayeNo ratings yet
- Republic of the Philippines College of Nursing Drug StudyDocument5 pagesRepublic of the Philippines College of Nursing Drug StudyChelsea WuNo ratings yet
- Burn - Concept MapDocument1 pageBurn - Concept MapAaron RafaelNo ratings yet
- Paracetamol Biogesic Analgesic AntipyreticDocument8 pagesParacetamol Biogesic Analgesic AntipyreticGian Era100% (1)
- Verapamil HCLDocument3 pagesVerapamil HCLMae Ann Bueno CastillonNo ratings yet
- Vitamin KDocument2 pagesVitamin KMuvs RazonNo ratings yet
- Drug Study - CefradoxilDocument13 pagesDrug Study - CefradoxilJohara G'naid0% (1)
- Ranitidine Generic for Zantac Reduces Stomach AcidDocument3 pagesRanitidine Generic for Zantac Reduces Stomach AcidMarychen Cabunas100% (1)
- AnastrozoleDocument2 pagesAnastrozoleAnonymous FgT04krgymNo ratings yet
- "Nahadlok Naman Ko Sa Akong Gipambati, Ning-Undang Ko Sakong Work As QHSE and Training Manager, Nagdecide Ko Muuli Sa Pilipinas. Pag-Uli Nako Last Week, Ginabati Nako Mura Ko Makulbaan" AsDocument4 pages"Nahadlok Naman Ko Sa Akong Gipambati, Ning-Undang Ko Sakong Work As QHSE and Training Manager, Nagdecide Ko Muuli Sa Pilipinas. Pag-Uli Nako Last Week, Ginabati Nako Mura Ko Makulbaan" Ashanna caballoNo ratings yet
- Name of Drug Classification Mechanism of Action Indication Contraindication Side Effects Nursing ResponsibilitiesDocument4 pagesName of Drug Classification Mechanism of Action Indication Contraindication Side Effects Nursing ResponsibilitiesMinaNo ratings yet
- Drug Study on CelecoxibDocument11 pagesDrug Study on CelecoxibPrincess Brigitte R. PATE�ANo ratings yet
- Timolol MaleateDocument3 pagesTimolol MaleateAP TOROBXNo ratings yet
- Drug Study - GDM - Caltrate PlusDocument2 pagesDrug Study - GDM - Caltrate PlusGAYOL BREEN IRAH A.No ratings yet
- Mosegor Vita Is A Vitamin SupplementDocument1 pageMosegor Vita Is A Vitamin SupplementlolabayNo ratings yet
- Albendazole - Drug Information PDFDocument7 pagesAlbendazole - Drug Information PDFjjjkkNo ratings yet
- Chn-Herbal MedicineDocument5 pagesChn-Herbal MedicineBSN 1-N CASTRO, RicciNo ratings yet
- PHINMA Nursing Drug StudyDocument2 pagesPHINMA Nursing Drug StudyArianne NicoleNo ratings yet
- TB DrugsDocument14 pagesTB DrugsLexy CadigalNo ratings yet
- Tramadol and Ciprofloxacin: Key Information for NursesDocument2 pagesTramadol and Ciprofloxacin: Key Information for NursesatchiekNo ratings yet
- Cefoxitin Sodium MefoxinDocument3 pagesCefoxitin Sodium MefoxinKristi WrayNo ratings yet
- BETAXOLOLDocument2 pagesBETAXOLOLjulieNo ratings yet
- Drug Study AtropineDocument3 pagesDrug Study AtropineAerron Severus Secano ShuldbergNo ratings yet
- Drug Study: Atropine: RecommendedDocument6 pagesDrug Study: Atropine: RecommendedShara Lailanie A. AzisNo ratings yet
- DroperidolDocument1 pageDroperidolIvanne HisolerNo ratings yet
- Drug Name WPS OfficeDocument2 pagesDrug Name WPS OfficeCAMILLE GAIL HADJIRANINo ratings yet
- Clozapine Nursing ResponsibilitiesDocument2 pagesClozapine Nursing ResponsibilitiesJvWoodzNo ratings yet
- Drug Study 20Document2 pagesDrug Study 20Alexandra AntondyNo ratings yet
- Drug Study 16Document3 pagesDrug Study 16Alexandra AntondyNo ratings yet
- MannitolDocument3 pagesMannitolAlexandra AntondyNo ratings yet
- 3 Places To Visit in Dapitan CityDocument2 pages3 Places To Visit in Dapitan CityAlexandra AntondyNo ratings yet
- FurosemidDocument3 pagesFurosemidAlexandra AntondyNo ratings yet
- FurosemidDocument3 pagesFurosemidAlexandra AntondyNo ratings yet
- AcetazolamideDocument2 pagesAcetazolamideAlexandra Antondy0% (1)
- GARCIA FAMILY CASE STUDY ANALYSISDocument1 pageGARCIA FAMILY CASE STUDY ANALYSISAlexandra AntondyNo ratings yet
- College of Nusrsing: Mindanao State University Iligan Institute of Technology Iligan City, 9200 PhilippinesDocument6 pagesCollege of Nusrsing: Mindanao State University Iligan Institute of Technology Iligan City, 9200 PhilippinesAlexandra AntondyNo ratings yet