Professional Documents
Culture Documents
Dilution Problems Worksheet
Uploaded by
osama0 ratings0% found this document useful (0 votes)
343 views1 pageThis document provides 7 chemistry problems involving calculating molarity (M), moles of solute, volume (V), or mass of solute given other variables using the molarity equation M1V1=M2V2. The problems cover diluting stock solutions to prepare solutions of specific molarities and volumes, calculating moles or mass of solute required for a given molarity and volume, and calculating molarity given moles or mass of solute and volume.
Original Description:
Original Title
Dilution Problems Worksheet.docx
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document provides 7 chemistry problems involving calculating molarity (M), moles of solute, volume (V), or mass of solute given other variables using the molarity equation M1V1=M2V2. The problems cover diluting stock solutions to prepare solutions of specific molarities and volumes, calculating moles or mass of solute required for a given molarity and volume, and calculating molarity given moles or mass of solute and volume.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
343 views1 pageDilution Problems Worksheet
Uploaded by
osamaThis document provides 7 chemistry problems involving calculating molarity (M), moles of solute, volume (V), or mass of solute given other variables using the molarity equation M1V1=M2V2. The problems cover diluting stock solutions to prepare solutions of specific molarities and volumes, calculating moles or mass of solute required for a given molarity and volume, and calculating molarity given moles or mass of solute and volume.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
Dilution Problems Worksheet (M 1 V 1 = M 2 V 2)
1. How much of a 15.0 M stock solution do you need to prepare 250 ml of a 2.35 M HF solution?
2. If 455 ml of 6.0 M HNO3 is diluted to 2.5 L, what is the molarity of the diluted solution?
3. If 65.5 ml of HCl stock solution is used to make 450 ml of a 0.675 M HCl dilution, what is the
molarity of the stock solution?
4. How do you prepare 500 ml of a 1.77 M H 2 SO 4 solution from an 18.0 M H 2 SO 4 stock solution?
Extra Molarity Problems for Practice
5. How many moles of LiF would be required to produce a 2.5 M solution with a volume of 1.5 L?
6. How many moles of Sr(NO3)2 would be used in the preparation of 2.50 L of a 3.5 M solution?
7. What is the molarity of a 500-ml solution containing 249 g of KI?
8. How many grams of CaCl2 would be required to produce a 3.5 M solution with a volume of 2.0 L?
You might also like
- Molarity and Dilution WorksheetsDocument2 pagesMolarity and Dilution Worksheetspearlparfait100% (2)
- Molarity & Dilution RVWDocument2 pagesMolarity & Dilution RVWkclyn escondoNo ratings yet
- Preparing, Diluting and Calculating Molarity of Solution: Chapter 2: Buffers and SolutionsDocument2 pagesPreparing, Diluting and Calculating Molarity of Solution: Chapter 2: Buffers and SolutionssyuhadahNo ratings yet
- Solutions Practice and DilutionsDocument2 pagesSolutions Practice and DilutionsBenjaminNo ratings yet
- Dilution Practice 2019Document1 pageDilution Practice 2019api-283677111No ratings yet
- Molarity Practice Problems 2Document2 pagesMolarity Practice Problems 2Pratik UpadhyayNo ratings yet
- 4.5 Concentrations of SolutionsDocument4 pages4.5 Concentrations of Solutionsjunkhead254No ratings yet
- 37 MolaritywebDocument10 pages37 MolaritywebpenisNo ratings yet
- RthyyDocument19 pagesRthyyXazerco LaxNo ratings yet
- Dilutions WorksheetDocument4 pagesDilutions WorksheetAtulya BharadwajNo ratings yet
- Dilution S WorksheetDocument4 pagesDilution S WorksheetZoe MarokoNo ratings yet
- Dilutions WorksheetDocument4 pagesDilutions WorksheetAtulya BharadwajNo ratings yet
- Calculation in AC-2Document36 pagesCalculation in AC-223005852No ratings yet
- Calculation in ACDocument4 pagesCalculation in AC23005852No ratings yet
- Tutorial On Laboratory CalculationDocument2 pagesTutorial On Laboratory Calculation13bellsNo ratings yet
- Chemistry - XI Topic: Some Basic Concepts of ChemistryDocument1 pageChemistry - XI Topic: Some Basic Concepts of ChemistryGurneetNo ratings yet
- Dilutions Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedDocument2 pagesDilutions Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedMayra FlorNo ratings yet
- Concentrations of Solutions Problem SolvingDocument2 pagesConcentrations of Solutions Problem SolvingJoe Ronnie TorresNo ratings yet
- Mole Conversions and Stoichiometry Review Worksheet: Find The Molarity of The Following SolutionsDocument3 pagesMole Conversions and Stoichiometry Review Worksheet: Find The Molarity of The Following SolutionsIsabelle ValeraNo ratings yet
- Concentrations of Solutions PracticeDocument1 pageConcentrations of Solutions PracticeJulia Geonzon LabajoNo ratings yet
- Molality and Colligative Properties Homework ChemistryDocument2 pagesMolality and Colligative Properties Homework Chemistrymary ann navarraNo ratings yet
- Seatwork 1Document1 pageSeatwork 1Drusilla Loss67% (3)
- Chemistry WorksheetDocument6 pagesChemistry WorksheetRashida TahaNo ratings yet
- Dilutions Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedDocument2 pagesDilutions Worksheet: © 2004 Cavalcade Publishing, All Rights Reservedchrimill69No ratings yet
- Revision: Moles, Solutions and Titration: Unit 1: Tutorial Series Module 1: Fundamentals in ChemistryDocument2 pagesRevision: Moles, Solutions and Titration: Unit 1: Tutorial Series Module 1: Fundamentals in ChemistryMel ManningNo ratings yet
- Name: - Date - PeriodDocument1 pageName: - Date - PeriodzaneNo ratings yet
- Concentration ProblemsDocument1 pageConcentration ProblemsmamazookeeprNo ratings yet
- Molarity Problems WorksheetDocument1 pageMolarity Problems WorksheetTeraGamingNo ratings yet
- Mole Calculations WorksheetDocument1 pageMole Calculations WorksheetNaeem ShayanNo ratings yet
- Titration Problem Set PDFDocument3 pagesTitration Problem Set PDFDianne Kate CadioganNo ratings yet
- ادامه تمرین های محلولهاDocument2 pagesادامه تمرین های محلولهاapi-3706290No ratings yet
- Practice Problems For Expressing ConcentrationsDocument4 pagesPractice Problems For Expressing ConcentrationsPamela ElumbaNo ratings yet
- CH 4 Chem 103 Brown 2011-2012Document12 pagesCH 4 Chem 103 Brown 2011-2012Shatha AlawnehNo ratings yet
- Molarity Lesson and Homework 2022Document1 pageMolarity Lesson and Homework 2022neliwok622No ratings yet
- Chemistry 30 - 2.2 - Concentration - WorksheetDocument1 pageChemistry 30 - 2.2 - Concentration - WorksheetKevin RamiroNo ratings yet
- Mola LarDocument2 pagesMola LarAshletyBultonNo ratings yet
- Level 1: The Program of Midterm Exam For Master Students 6M060600 - "Chemistry"Document2 pagesLevel 1: The Program of Midterm Exam For Master Students 6M060600 - "Chemistry"LOREI FELISSE GARNACENo ratings yet
- Quiz Laboratory MathematicsDocument1 pageQuiz Laboratory MathematicsFedz FederisoNo ratings yet
- Exercises - SolutionsDocument1 pageExercises - Solutionsgnye20No ratings yet
- Soln WS More PracticeDocument3 pagesSoln WS More PracticePauloM.RejusoNo ratings yet
- Dilution Problem Solving PDFDocument8 pagesDilution Problem Solving PDFลูซี้หมี ขวัญใจ ปัญญาสรกุลNo ratings yet
- Molality and Molarity WorksheetDocument1 pageMolality and Molarity WorksheetKenneth Roy MatuguinaNo ratings yet
- AP Chemistry: Solution Stoichiometry Practice ProblemsDocument4 pagesAP Chemistry: Solution Stoichiometry Practice ProblemsTutor AcademyNo ratings yet
- Dry Lab Activity No. 2 Concentration DilutionDocument4 pagesDry Lab Activity No. 2 Concentration DilutionAllen ChristianNo ratings yet
- Pset 1 Solutions-1 PDFDocument1 pagePset 1 Solutions-1 PDFMary AlcantaraNo ratings yet
- Tutorial 1 (Chapter 1: Introduction To Analytical Chemistry) CHM 256Document2 pagesTutorial 1 (Chapter 1: Introduction To Analytical Chemistry) CHM 256intanNo ratings yet
- Lab Report 1 (Bio462)Document5 pagesLab Report 1 (Bio462)Allisya NasirNo ratings yet
- Titrations Practice ProblemsDocument3 pagesTitrations Practice ProblemsbenitonziratimaanaNo ratings yet
- Topic 2 Exercise 2 - SolutionsDocument2 pagesTopic 2 Exercise 2 - SolutionsSalman ZaidiNo ratings yet
- Chemistry II Blizzard Bag #3 Concentration WorksheetDocument1 pageChemistry II Blizzard Bag #3 Concentration Worksheetapi-239855791No ratings yet
- Chemistry Concepts Stoichiometry Water SDocument50 pagesChemistry Concepts Stoichiometry Water SJoseph DakaNo ratings yet
- Molarity Practice WorksheetDocument1 pageMolarity Practice WorksheetAshnie RaghnauthNo ratings yet
- Introduction To Molarity: High Concentration Indicates A Low Concentration Indicates ADocument10 pagesIntroduction To Molarity: High Concentration Indicates A Low Concentration Indicates AGrace BatlleNo ratings yet
- Achem Sample ProblemsDocument2 pagesAchem Sample ProblemsCharles Daniel Torre MalolesNo ratings yet
- Tutorial Chm256 Chapter1 (Part 2)Document2 pagesTutorial Chm256 Chapter1 (Part 2)Siti Suhailah100% (1)
- Ejercicios de Concentración en Disoluciones 1Document2 pagesEjercicios de Concentración en Disoluciones 1Anonymous L8cgq9No ratings yet
- Assessmenmt Test # - 2Document2 pagesAssessmenmt Test # - 2Aiza VelascoNo ratings yet
- Practice Exercises (Molarity) PDFDocument10 pagesPractice Exercises (Molarity) PDFKenneth Roy MatuguinaNo ratings yet
- The Chemistry of Dairy Products - A Chemical Analysis of Milk, Cream and ButterFrom EverandThe Chemistry of Dairy Products - A Chemical Analysis of Milk, Cream and ButterNo ratings yet
- Forms of Energy - Print - QuizizzDocument6 pagesForms of Energy - Print - Quizizzosama100% (1)
- Writing A Lab ReportDocument26 pagesWriting A Lab ReportosamaNo ratings yet
- Lesson 3 - Moving Cellular Material: Student Labs and Activities Appropriate ForDocument20 pagesLesson 3 - Moving Cellular Material: Student Labs and Activities Appropriate ForosamaNo ratings yet
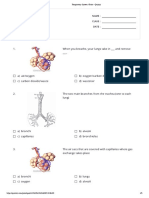
- Respiratory System - Print - QuizizzDocument5 pagesRespiratory System - Print - QuizizzosamaNo ratings yet
- Read To LearnDocument5 pagesRead To LearnosamaNo ratings yet
- Calculating Acceleration - Print - QuizizzDocument6 pagesCalculating Acceleration - Print - QuizizzosamaNo ratings yet
- Chapter 5 - Energy Notes - CompleteDocument22 pagesChapter 5 - Energy Notes - CompleteosamaNo ratings yet
- Respiratory System - Print - QuizizzDocument5 pagesRespiratory System - Print - QuizizzosamaNo ratings yet
- The Heart - Print - QuizizzDocument5 pagesThe Heart - Print - Quizizzosama100% (1)
- Cell Transport & Homeostasis - Print - QuizizzDocument3 pagesCell Transport & Homeostasis - Print - Quizizzosama100% (1)
- Cut Out The Pictures and Stick Them On in The Right OrderDocument3 pagesCut Out The Pictures and Stick Them On in The Right OrderosamaNo ratings yet
- Chapter 5 Energy Review - Print - QuizizzDocument5 pagesChapter 5 Energy Review - Print - QuizizzosamaNo ratings yet
- Kinetic Energy Worksheet: The Energy Due To MotionDocument1 pageKinetic Energy Worksheet: The Energy Due To MotionosamaNo ratings yet
- Section Quick Check Organization of The Nervous System EditableDocument2 pagesSection Quick Check Organization of The Nervous System EditableosamaNo ratings yet
- Cell Theory and Types of Microscopes - Print - QuizizzDocument4 pagesCell Theory and Types of Microscopes - Print - QuizizzosamaNo ratings yet
- Study Guide: Section 2: The Respiratory SystemDocument3 pagesStudy Guide: Section 2: The Respiratory SystemosamaNo ratings yet
- Study Guide: Section 1: The Circulatory SystemDocument4 pagesStudy Guide: Section 1: The Circulatory SystemosamaNo ratings yet
- Excretory System - Print - QuizizzDocument4 pagesExcretory System - Print - Quizizzosama0% (1)
- Lesson 3 Classifying OrganismsDocument33 pagesLesson 3 Classifying OrganismsosamaNo ratings yet
- Research SampleDocument11 pagesResearch SampleosamaNo ratings yet
- Concept Mapping Circulatory Respiratory and Excretory Systems EditableDocument2 pagesConcept Mapping Circulatory Respiratory and Excretory Systems EditableosamaNo ratings yet
- Microscope LabelingDocument1 pageMicroscope LabelingosamaNo ratings yet
- Study Guide: Section 1: Animal CharacteristicsDocument5 pagesStudy Guide: Section 1: Animal CharacteristicsosamaNo ratings yet
- Biology H.W 11.2Document1 pageBiology H.W 11.2osamaNo ratings yet
- Week 5 Research Skills and CitationDocument7 pagesWeek 5 Research Skills and CitationosamaNo ratings yet
- Monohybrid Cross PhenotypeDocument2 pagesMonohybrid Cross PhenotypeosamaNo ratings yet
- Your Task For Today Is To Watch The 10 Minutes' Video Posted in Classera Under The NameDocument1 pageYour Task For Today Is To Watch The 10 Minutes' Video Posted in Classera Under The NameosamaNo ratings yet
- Reading Essentials For Biology (PDF - Io)Document5 pagesReading Essentials For Biology (PDF - Io)osamaNo ratings yet
- Domains and Kingdoms: Study GuideDocument6 pagesDomains and Kingdoms: Study GuideosamaNo ratings yet
- Worksheet 18.2 P A - R E: ART Eaction NergyDocument2 pagesWorksheet 18.2 P A - R E: ART Eaction NergyosamaNo ratings yet