Professional Documents
Culture Documents
Pages From 5054 - w15 - QP - 22-6 - Gas Pressure
Uploaded by
lelon ongOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Pages From 5054 - w15 - QP - 22-6 - Gas Pressure
Uploaded by
lelon ongCopyright:
Available Formats
9
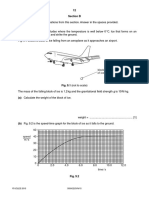
6 A gas is trapped at atmospheric pressure in a cylinder by a piston. The piston is held in a fixed
position by a movable rod. Fig. 6.1 shows the cylinder.
trapped gas
piston
cylinder
rod
Fig. 6.1
The cylinder is heated. As the temperature of the gas increases, its pressure increases.
(a) Explain, in terms of molecules, why the pressure of the trapped gas increases.
...................................................................................................................................................
...................................................................................................................................................
...................................................................................................................................................
.............................................................................................................................................. [2]
(b) The rod is pulled down and the piston is then free to move as shown in Fig. 6.2.
trapped gas
piston
cylinder
rod
Fig. 6.2
As the piston moves, the temperature of the gas remains constant.
State and explain, in terms of molecules, what happens to the pressure of the gas.
...................................................................................................................................................
...................................................................................................................................................
...................................................................................................................................................
...................................................................................................................................................
...................................................................................................................................................
.............................................................................................................................................. [3]
© UCLES 2015 5054/22/O/N/15 [Turn over
You might also like
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Omoda 5 BrochureDocument4 pagesOmoda 5 Brochurelelon ongNo ratings yet
- Pages From 0625 - w15 - QP - 31-10Document2 pagesPages From 0625 - w15 - QP - 31-10lelon ongNo ratings yet
- Pages From 0625 - w15 - QP - 31-09Document1 pagePages From 0625 - w15 - QP - 31-09lelon ongNo ratings yet
- Chapter 67-2Document12 pagesChapter 67-2lelon ongNo ratings yet
- Pages From 0625 - w15 - QP - 32-10Document2 pagesPages From 0625 - w15 - QP - 32-10lelon ong0% (1)
- Pages From 0625 - w15 - QP - 31-10Document2 pagesPages From 0625 - w15 - QP - 31-10lelon ongNo ratings yet
- Pages From 0625 - w15 - QP - 31-08 PDFDocument1 pagePages From 0625 - w15 - QP - 31-08 PDFlelon ongNo ratings yet
- Pages From 0625 - w15 - QP - 31-06 PDFDocument1 pagePages From 0625 - w15 - QP - 31-06 PDFlelon ongNo ratings yet
- Pages From 0625 - w15 - QP - 33-06Document2 pagesPages From 0625 - w15 - QP - 33-06lelon ongNo ratings yet
- Pages From 0625 - w15 - QP - 32-08Document2 pagesPages From 0625 - w15 - QP - 32-08lelon ongNo ratings yet
- Pages From 0625 - w15 - QP - 31-07Document2 pagesPages From 0625 - w15 - QP - 31-07lelon ongNo ratings yet
- Pages From 5054 - w15 - QP - 22-5 - ThermometerDocument1 pagePages From 5054 - w15 - QP - 22-5 - Thermometerlelon ongNo ratings yet
- Pages From 0625 - w15 - QP - 32-07Document2 pagesPages From 0625 - w15 - QP - 32-07lelon ongNo ratings yet
- Pages From 5054 - w15 - QP - 22-9 - Chapter Kinematic and ThermalDocument3 pagesPages From 5054 - w15 - QP - 22-9 - Chapter Kinematic and Thermallelon ongNo ratings yet
- Pages From 5054 - w15 - QP - 22-6 - Gas PressureDocument1 pagePages From 5054 - w15 - QP - 22-6 - Gas Pressurelelon ongNo ratings yet
- Pages From 0625 - w15 - QP - 32-05Document1 pagePages From 0625 - w15 - QP - 32-05lelon ongNo ratings yet
- Pages From 0625 - w15 - QP - 33-05Document1 pagePages From 0625 - w15 - QP - 33-05lelon ongNo ratings yet
- Pages From 0625 - w15 - QP - 33-05Document1 pagePages From 0625 - w15 - QP - 33-05lelon ongNo ratings yet
- Pages From 5054 - w15 - QP - 22-4 - Thermal TransferDocument1 pagePages From 5054 - w15 - QP - 22-4 - Thermal Transferlelon ongNo ratings yet
- Pages From 5054 - w15 - QP - 22-5 - ThermometerDocument1 pagePages From 5054 - w15 - QP - 22-5 - Thermometerlelon ongNo ratings yet
- Pages From 5054 - w15 - QP - 22-4 - Thermal TransferDocument1 pagePages From 5054 - w15 - QP - 22-4 - Thermal Transferlelon ongNo ratings yet
- Pages From 0625 - w15 - QP - 32-06Document1 pagePages From 0625 - w15 - QP - 32-06lelon ongNo ratings yet
- Pages From 5054 - w15 - QP - 22-2 - Hookes LawDocument1 pagePages From 5054 - w15 - QP - 22-2 - Hookes Lawlelon ongNo ratings yet
- Pages From 0625 - w15 - QP - 31-05Document1 pagePages From 0625 - w15 - QP - 31-05lelon ongNo ratings yet
- Pages From 0625 - w15 - QP - 31-04Document1 pagePages From 0625 - w15 - QP - 31-04lelon ongNo ratings yet
- Pages From 5054 - w15 - QP - 22-3 - PressureDocument2 pagesPages From 5054 - w15 - QP - 22-3 - Pressurelelon ongNo ratings yet
- Pages From 5054 - w15 - QP - 21-4 - MomentDocument1 pagePages From 5054 - w15 - QP - 21-4 - Momentlelon ongNo ratings yet
- Pages From 5054 - w15 - QP - 21-9 - Chapter Kinematic and Vector DiagramDocument2 pagesPages From 5054 - w15 - QP - 21-9 - Chapter Kinematic and Vector Diagramlelon ongNo ratings yet
- Pages From 5054 - w15 - QP - 21-9 - Chapter Kinematic and Vector DiagramDocument2 pagesPages From 5054 - w15 - QP - 21-9 - Chapter Kinematic and Vector Diagramlelon ongNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- EECS4421Z: Introduction To Robotics Sample Exam QuestionsDocument7 pagesEECS4421Z: Introduction To Robotics Sample Exam QuestionsMooeez BellaamineNo ratings yet
- Free Electricity From The Earth Rotating Through Its Own Magnetic Field Using The Homopolar Generator EffectDocument3 pagesFree Electricity From The Earth Rotating Through Its Own Magnetic Field Using The Homopolar Generator EffectmarcelogiovaneNo ratings yet
- Mariners' Polytechnic Colleges Foundation of Canaman, Camarines SurDocument4 pagesMariners' Polytechnic Colleges Foundation of Canaman, Camarines SurKristian-Emman SarateNo ratings yet
- Monitoreo de BombasDocument6 pagesMonitoreo de Bombasroberdani12No ratings yet
- Coding Bobol ExcelDocument4 pagesCoding Bobol ExcelMuhammad IsmunandarsyahNo ratings yet
- Reading A Film - Problem of Denotation in Fiction FilmDocument0 pagesReading A Film - Problem of Denotation in Fiction FilmragatasuryaNo ratings yet
- Ijtmsr201919 PDFDocument5 pagesIjtmsr201919 PDFPrakash InturiNo ratings yet
- 2.lecture 1-Basics and PrecedenceDocument30 pages2.lecture 1-Basics and PrecedenceBhavesh ReddyNo ratings yet
- Functions Equations Question BankDocument101 pagesFunctions Equations Question BankParth DesaiNo ratings yet
- StepperDocument7 pagesStepperahmad_syafrudin_1No ratings yet
- Denture Base MaterialsDocument117 pagesDenture Base MaterialsLalit KumarNo ratings yet
- Biology Transportation in PlantsDocument6 pagesBiology Transportation in PlantsTanaka ChirawuNo ratings yet
- 2200SRM1266 (06 2006) Uk enDocument28 pages2200SRM1266 (06 2006) Uk enEbied Yousif Aly100% (9)
- How Torque Converters Work - HowStuffWorksDocument7 pagesHow Torque Converters Work - HowStuffWorksKrishanu ModakNo ratings yet
- DWSIM Training-V02-30dec17Document58 pagesDWSIM Training-V02-30dec17Zanariah HashimNo ratings yet
- Department of Mechanical Engineering Question Bank Subject Name: Heat & Mass Transfer Unit - I Conduction Part - ADocument3 pagesDepartment of Mechanical Engineering Question Bank Subject Name: Heat & Mass Transfer Unit - I Conduction Part - AkarthikNo ratings yet
- Flanges: SI SMEDocument16 pagesFlanges: SI SMEbalaNo ratings yet
- Family TreeDocument5 pagesFamily Treeddkunkun267No ratings yet
- Chapter 20 Surface Area and Volume of A Right Circular ConeDocument19 pagesChapter 20 Surface Area and Volume of A Right Circular ConeMann GosarNo ratings yet
- Methods of Collecting DataDocument26 pagesMethods of Collecting DataLolol LololNo ratings yet
- Thermal Engineering For The Construction of Large Concrete Arch DamsDocument10 pagesThermal Engineering For The Construction of Large Concrete Arch DamsOscar LopezNo ratings yet
- 235practice Exam 2 AnswerDocument9 pages235practice Exam 2 Answernbobs7No ratings yet
- SmartPRO 5000U Plus ManualDocument10 pagesSmartPRO 5000U Plus ManualMugiranezaNo ratings yet
- DDP400 Open-Frame and U-Chassis :: ROAL Living EnergyDocument12 pagesDDP400 Open-Frame and U-Chassis :: ROAL Living EnergyroalscribdNo ratings yet
- TECNICA 140.1 - 142 TECNICA 1400-1600: Inver TerDocument20 pagesTECNICA 140.1 - 142 TECNICA 1400-1600: Inver TerabdessNo ratings yet
- How To Implement A Distributed CommonJ Work Manager ESDocument20 pagesHow To Implement A Distributed CommonJ Work Manager ESAbmel Salim LopessierNo ratings yet
- Boxer EngineDocument84 pagesBoxer EngineTOONGA100% (7)
- BasCal (1st Long Exam Reviewer)Document23 pagesBasCal (1st Long Exam Reviewer)Ethan Erika BionaNo ratings yet
- Jawaharlal Nehru Technological University Anantapur B.Tech. I - I Sem. L C 4 2 Part-A LABDocument2 pagesJawaharlal Nehru Technological University Anantapur B.Tech. I - I Sem. L C 4 2 Part-A LABHappa1No ratings yet