Professional Documents
Culture Documents
Absorbancecoefficient PDF
Uploaded by
kofinyameOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Absorbancecoefficient PDF
Uploaded by
kofinyameCopyright:
Available Formats
3B3: Calculating the molar absorbance coefficient from experimental data
3.B.3. Calculating the molar absorbance coefficient (ε) from absorbance
and concentration data
.
Learning Objective.
To calculate a value for ε from experimental data of absorbance and concentration.
.
In this example we are using data for p-nitrophenol which is a yellow-coloured reagent
commonly used in diagnostic tests (ELISA’s).
p-nitrophenol absorbs well with light of about 400 nm so we measure the absorbance
using light of that wavelength in a cuvette of pathlength 1 cm and call the absorbance A400.
.
plot the data on a graph of A vs C
set up some cuvettes measure the
containing a range of absorbance for each
concentrations of p- cuvette 1
0.9
nitrophenol from 0 to 0.05 mM C (mM) A400
0.8
0.7
0 0 400 0.6
0.5
A
0.01 0.18 0.4
0.3
0.02 0.37 0.2
0.03 0.55 0.1
C (mM) = 0 0.01 0.02 0.03 0.04 0.05 0
0.04 0.72 0 0.01 0.02 0.03 0.04 0.05
0.05 0.91 C (mM)
A = εCd = (εd)C; in a graph of A vs C, the slope is εd.
y2 − y1 0.91 − 0
= 18.2(mM )
−1
slope = ε .d = =
x2 − x1 (0.05 − 0 )mM
d = 1 cm
so
18.2(mM )
−1
18.2(mM )
−1
ε= = = 18.2mM −1cm −1
d 1cm
Creative Commons Attribution Non-commercial Share Alike
Author: Dr Jenny A Koenig Page 1 of 2
3B3: Calculating the molar absorbance coefficient from experimental data
However.... ε is usually written with the units M-1.cm-1.
How do we get ε in the right units?
Two possible methods:
1
Possibly the easiest way is to start with M rather than mM in the first place.
0.91 − 0
εd =
(0.05 − 0) × 10 −3 M
(
= 18.2 10 −3 M )
−1
= 18.2 × 10 3 M −1 = 18200 M −1
then
ε = 18200 M-1 / 1 cm = 18200 M-1.cm-1
.
2
Another method is to say
1000 mM = 1 M,
so 1000 mM.M-1 = 1
If ε = 18.2 mM-1.cm-1
then you can multiply both sides by 1 (=1000 mM.M-1)
ε = 18.2 mM-1.cm-1 x 1000 mM.M-1
then the mM-1 cancels with the mM
ε = 18.2 mM-1.cm-1 x 1000 mM.M-1
and you are left with
ε = 18200 M-1cm-1
.
Creative Commons Attribution Non-commercial Share Alike
Author: Dr Jenny A Koenig Page 2 of 2
You might also like
- 3 B 3 Print Able VersionDocument2 pages3 B 3 Print Able Versionfernanda boldtNo ratings yet
- Absorbancecoefficient PDFDocument2 pagesAbsorbancecoefficient PDFEduardo GarzaNo ratings yet
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportFrom EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportNo ratings yet
- Calculation of Molar AbsorbtivityDocument2 pagesCalculation of Molar AbsorbtivityTarek NajemNo ratings yet
- Analysis of Nitrite Ion Contents in Meat Sample by UV Vis Spectroscopy Method Results and DiscussionDocument2 pagesAnalysis of Nitrite Ion Contents in Meat Sample by UV Vis Spectroscopy Method Results and DiscussionANH NGUYỄN HUỲNH MINHNo ratings yet
- CV: Cyclic Voltammetry For The Determination of The Concentration of Ferrocyanide/Ferricyanide Redox Couple. Maha Zerkan 02/03/2022Document7 pagesCV: Cyclic Voltammetry For The Determination of The Concentration of Ferrocyanide/Ferricyanide Redox Couple. Maha Zerkan 02/03/2022Maha ZerkanNo ratings yet
- CALCULATIONSDocument3 pagesCALCULATIONSgoabaone kgopaNo ratings yet
- Perhitungan: Mol M X LDocument10 pagesPerhitungan: Mol M X Lfitrah fajrianiNo ratings yet
- Lab Report (Atomic Absorption Spectroscopy)Document8 pagesLab Report (Atomic Absorption Spectroscopy)Shirley Cheong67% (6)
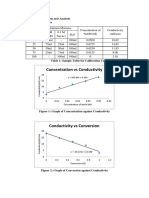
- Concentration Vs Conductivity: 1.0 Results, Discussion and Analysis 1.1 Calibration CurveDocument9 pagesConcentration Vs Conductivity: 1.0 Results, Discussion and Analysis 1.1 Calibration CurveAhZaiSkyNo ratings yet
- Lecture 15 16Document28 pagesLecture 15 16aadhyaNo ratings yet
- Lab Report 03 - Edm CalibrationDocument2 pagesLab Report 03 - Edm CalibrationShivam ShuklaNo ratings yet
- Bridging The Chasm ActivityDocument6 pagesBridging The Chasm Activityapi-616433899No ratings yet
- Problem GaoDocument3 pagesProblem Gaom.a.saeedNo ratings yet
- Concentration vs. Absorbance: 1. Standard CurveDocument2 pagesConcentration vs. Absorbance: 1. Standard CurveHee MinNo ratings yet
- Measurement of Magnetic Field Inside A Solenoid With Finite LengthDocument5 pagesMeasurement of Magnetic Field Inside A Solenoid With Finite LengthnamNo ratings yet
- Determination of Mixtures by UV Absorption Spectroscopy: Chem 155 Lab Dr. TerrillDocument8 pagesDetermination of Mixtures by UV Absorption Spectroscopy: Chem 155 Lab Dr. Terrillkir223No ratings yet
- Choba 408 EXP 2Document12 pagesChoba 408 EXP 2Choba Tapaphiwa ChobaNo ratings yet
- PBRDocument19 pagesPBRdarvyneeNo ratings yet
- Sara LaFreniere P115 B43 Lab ReportDocument7 pagesSara LaFreniere P115 B43 Lab ReportMickey AngeloNo ratings yet
- Experiment.4 Uv/Vis Spectrophotometry: Department of Chemistry University of Bahrain CHEMY310Document6 pagesExperiment.4 Uv/Vis Spectrophotometry: Department of Chemistry University of Bahrain CHEMY310Zahra Al-BasriNo ratings yet
- Exercise 1: Spectrochemical Analysis: BE132P Instrumentation in Biological Engineering 1Document7 pagesExercise 1: Spectrochemical Analysis: BE132P Instrumentation in Biological Engineering 1Bernadette Virola CuevasNo ratings yet
- Supplementary MaterialDocument7 pagesSupplementary MaterialInigo JohnsonNo ratings yet
- Assignment 03 - Chapter 3 PDFDocument3 pagesAssignment 03 - Chapter 3 PDFChanten NanNo ratings yet
- Report 12 InstrumentalDocument3 pagesReport 12 InstrumentalKim Yến PhùngNo ratings yet
- Determination of Composition of Complexes Using Jobs Method (1) NoDocument10 pagesDetermination of Composition of Complexes Using Jobs Method (1) NoCh Safdar FarukhNo ratings yet
- CalculationsDocument6 pagesCalculationsJoseph FrengleyNo ratings yet
- Experiment-2: Shear Centre of Open SectionsDocument7 pagesExperiment-2: Shear Centre of Open SectionsRahul RoyNo ratings yet
- PHY 2042 Experiment #10Document2 pagesPHY 2042 Experiment #10Kelsey WNo ratings yet
- Determination of The Acetaminophen Concentration in An Elixir Using Cyclic VoltammetryDocument9 pagesDetermination of The Acetaminophen Concentration in An Elixir Using Cyclic VoltammetryMikahNo ratings yet
- NCHE312Document11 pagesNCHE312Charmaine MoyoNo ratings yet
- Circular Motion: Physics Department Electricity and Magnetism LaboratoryDocument12 pagesCircular Motion: Physics Department Electricity and Magnetism LaboratoryBeatriz IzquierdoNo ratings yet
- Shear Force and Bending Moment Influence Line - Group 1 - Section 4Document19 pagesShear Force and Bending Moment Influence Line - Group 1 - Section 4Muhammad Hazim Bin Ahmad FauziNo ratings yet
- Experiments PDFDocument5 pagesExperiments PDFSuryansh KhatiNo ratings yet
- HW 4 A 1Document11 pagesHW 4 A 1draconnoxNo ratings yet
- Exercises of Analytical Chemistry - Part 1: A. Stoichiometry ReminderDocument16 pagesExercises of Analytical Chemistry - Part 1: A. Stoichiometry ReminderTeresa RiosNo ratings yet
- Results:: A) Experimental Shear CentreDocument4 pagesResults:: A) Experimental Shear Centrekaram bawadiNo ratings yet
- Iodine LabDocument4 pagesIodine LabHuang ViviNo ratings yet
- E - M OF ELECTRON LAB PDFDocument9 pagesE - M OF ELECTRON LAB PDFAisha AtkinsonNo ratings yet
- R V S/ (S + K) : Computer Methods and Programs in Biomedicine 65, 191-200Document10 pagesR V S/ (S + K) : Computer Methods and Programs in Biomedicine 65, 191-200Lucas Hernández Karla BereniceNo ratings yet
- Experimental Report 2-CompletedDocument4 pagesExperimental Report 2-CompletedKiệt Như LêNo ratings yet
- ví dụ xác định đường đẳng nhiệt hấp phụDocument2 pagesví dụ xác định đường đẳng nhiệt hấp phụTHƯ NGUYỄN THỊ MINHNo ratings yet
- End Point Energy of Beta Rays Using GM TubeDocument4 pagesEnd Point Energy of Beta Rays Using GM TubeSanjana DondadkarNo ratings yet
- Index Notation PracticeDocument6 pagesIndex Notation PracticeSebastian hanNo ratings yet
- Chemy 310 Experiment 5Document9 pagesChemy 310 Experiment 5Faisal MumtazNo ratings yet
- Materials Science and Engineering An Introduction 9Th Edition Callister Solutions Manual Full Chapter PDFDocument67 pagesMaterials Science and Engineering An Introduction 9Th Edition Callister Solutions Manual Full Chapter PDFCarolineAvilakgjq100% (11)
- Chemistry 2a Form Iv Marking Scheme-1Document4 pagesChemistry 2a Form Iv Marking Scheme-1Mohammed B.S. MakimuNo ratings yet
- Adsorption Exp CalculationsDocument2 pagesAdsorption Exp CalculationsDilip Singh Choudhary100% (1)
- Design of Absorption ColumnDocument33 pagesDesign of Absorption ColumnJu Naid MalikNo ratings yet
- Homework 1 Student Answer WebCTDocument3 pagesHomework 1 Student Answer WebCTTsz Wun CHOWNo ratings yet
- Clinical Chemistry 1 (MKEB2404)Document10 pagesClinical Chemistry 1 (MKEB2404)kiedd_04100% (3)
- Result Exp Protein AssayDocument2 pagesResult Exp Protein AssayJaneNo ratings yet
- Calibration CurveDocument13 pagesCalibration Curvecinvehbi711No ratings yet
- Bui Viet Phuong HW11Document6 pagesBui Viet Phuong HW11Bùi Việt PhươngNo ratings yet
- Foster Cole 101230199 Malaïka Zarrouki 2021-01-29Document7 pagesFoster Cole 101230199 Malaïka Zarrouki 2021-01-29Cole FosterNo ratings yet
- Lembar Perhitungan ReagenDocument16 pagesLembar Perhitungan ReagenZoe SinulinggaNo ratings yet
- Lab AnalysisDocument4 pagesLab AnalysisErnestasBlaževičNo ratings yet
- 10 Percent Acid Washing SOP TemplateDocument5 pages10 Percent Acid Washing SOP TemplatekofinyameNo ratings yet
- 2nd Exp. Electronic Spectra - Cu in ComplexesDocument5 pages2nd Exp. Electronic Spectra - Cu in ComplexeskofinyameNo ratings yet
- 2.electronic Spectra of Cu (II) Complexes (Brief Procedure 16-10-20)Document1 page2.electronic Spectra of Cu (II) Complexes (Brief Procedure 16-10-20)kofinyameNo ratings yet
- Employee Satisfaction Survey Detailed Version PDFDocument5 pagesEmployee Satisfaction Survey Detailed Version PDFAleeza KhanNo ratings yet
- 1.bis (Acetylacetonato) Copper (II) (Brief Procedue16-10-20)Document1 page1.bis (Acetylacetonato) Copper (II) (Brief Procedue16-10-20)kofinyameNo ratings yet
- 1st Exp Prep and Analysis of Cu (Acac) 2Document5 pages1st Exp Prep and Analysis of Cu (Acac) 2kofinyameNo ratings yet
- Character Assasins in The Church-Part 1Document6 pagesCharacter Assasins in The Church-Part 1kofinyameNo ratings yet
- Once Safe, Always Safe............. The Case of Herbal MedicinesDocument2 pagesOnce Safe, Always Safe............. The Case of Herbal MedicineskofinyameNo ratings yet
- Regulation of Herbal Medicines in GhanaDocument2 pagesRegulation of Herbal Medicines in GhanakofinyameNo ratings yet
- Why The Nation Must Invest in Herbal Medicine DevelopmentDocument3 pagesWhy The Nation Must Invest in Herbal Medicine DevelopmentkofinyameNo ratings yet
- The Congo Job-Why Asante Kotoko Cannot Fail Their PatronsDocument2 pagesThe Congo Job-Why Asante Kotoko Cannot Fail Their PatronskofinyameNo ratings yet
- Doctor Patient RelationshipDocument4 pagesDoctor Patient RelationshipkofinyameNo ratings yet
- Character Assasins in The Church-Part 1Document6 pagesCharacter Assasins in The Church-Part 1kofinyameNo ratings yet
- What African Leaders Must Do To Harness TheDocument2 pagesWhat African Leaders Must Do To Harness ThekofinyameNo ratings yet
- Integration of Herbal Medicine in GhanaDocument4 pagesIntegration of Herbal Medicine in GhanakofinyameNo ratings yet
- 2.2: Limit of A Function and Limit Laws: Learning ObjectivesDocument16 pages2.2: Limit of A Function and Limit Laws: Learning ObjectiveskofinyameNo ratings yet
- APA Referencing GuideDocument4 pagesAPA Referencing GuidekofinyameNo ratings yet
- Definition 1: The Limit of A FunctionDocument6 pagesDefinition 1: The Limit of A FunctionkofinyameNo ratings yet
- Attachment and Delinquency PDFDocument15 pagesAttachment and Delinquency PDFkofinyameNo ratings yet
- SDS For Concentrated - Nitric - Acid - Nov - 2017Document10 pagesSDS For Concentrated - Nitric - Acid - Nov - 2017kofinyameNo ratings yet
- Guidelines For Maintenance of Equipment in A Clinical Chemistry LaboratoryDocument104 pagesGuidelines For Maintenance of Equipment in A Clinical Chemistry LaboratoryMuhammed Hunais83% (6)
- URIT-8021A Service Manual PDFDocument184 pagesURIT-8021A Service Manual PDFRichar Armando Timaran TumbacoNo ratings yet
- Mispa Nano Plus-1Document4 pagesMispa Nano Plus-1gouse bashaNo ratings yet
- Course Code: PHA 305 UV-Visible SpectrophotometryDocument38 pagesCourse Code: PHA 305 UV-Visible SpectrophotometryMahadi Hasan KhanNo ratings yet
- Ceebee 300LFDocument6 pagesCeebee 300LFHUANG StevenNo ratings yet
- Biochemistry Laboratory Manual Che 4350: Andrew J. Bonham, PH.D., Annamarie Drotar, PH.D., Kelly M. Elkins, Ph. DDocument79 pagesBiochemistry Laboratory Manual Che 4350: Andrew J. Bonham, PH.D., Annamarie Drotar, PH.D., Kelly M. Elkins, Ph. Daaron mbindyoNo ratings yet
- Indiko and Indiko Plus BrochureDocument8 pagesIndiko and Indiko Plus BrochuresharenNo ratings yet
- BSC BTDocument10 pagesBSC BTAayushmaan KumarNo ratings yet
- Stabilitas AspirinDocument9 pagesStabilitas AspirinHelenMonicaNo ratings yet
- OQPV PDA996 71550299614rBDocument28 pagesOQPV PDA996 71550299614rBComptoir ChromatoNo ratings yet
- Ist727 07 20Document2 pagesIst727 07 20aisyah annisa rahma hidayahNo ratings yet
- Ethanol: Assay ProcedureDocument16 pagesEthanol: Assay ProcedureCatalin OpreaNo ratings yet
- Biosystems Ba400 PDFDocument132 pagesBiosystems Ba400 PDFأنور مازوز أبو يوسف100% (2)
- Milton Roy Spectronic 21 ManualDocument50 pagesMilton Roy Spectronic 21 ManualBrendan Rackley88% (17)
- IB Chemistry HL Internal AssessmentDocument14 pagesIB Chemistry HL Internal AssessmentZinzan Gurney100% (1)
- BIOBASE Automatic Chemistry Analyzer BK-280 User Manual 202101Document147 pagesBIOBASE Automatic Chemistry Analyzer BK-280 User Manual 202101Raimundo BeltranNo ratings yet
- CP 13 - Growth of Microorganisms in Liquid CultureDocument4 pagesCP 13 - Growth of Microorganisms in Liquid Culturerifu91No ratings yet
- CuDocument11 pagesCuEbenezer EffisahNo ratings yet
- Hy-Lite 2 System: Operating Instructions ManualDocument33 pagesHy-Lite 2 System: Operating Instructions ManualIrfan NurdiansyahNo ratings yet
- Genesys Uv Vis Spectrophotometer Accessories Brochure BR52302Document12 pagesGenesys Uv Vis Spectrophotometer Accessories Brochure BR52302Norbey Marin MorenoNo ratings yet
- BOECO Spectrophotometer - Photometer 2014Document4 pagesBOECO Spectrophotometer - Photometer 2014Noel MamaniNo ratings yet
- Spectrophotometer/Fluorimeter: Cuvette SpecificationsDocument3 pagesSpectrophotometer/Fluorimeter: Cuvette Specificationsegyptian_scientistNo ratings yet
- Photometry: Chapter 3: Analytical TechniqueDocument6 pagesPhotometry: Chapter 3: Analytical TechniqueCL SanchezNo ratings yet
- URIT-880 User ManualDocument89 pagesURIT-880 User ManualHelen DiazNo ratings yet
- Dynamica Halo VIS-20 Instructional ManualDocument58 pagesDynamica Halo VIS-20 Instructional ManualDaniel VargasNo ratings yet
- BIO 462 Experiment 2Document6 pagesBIO 462 Experiment 2Nurul Farhah RadzuwanNo ratings yet
- Colorimeter Sensor DT185A: Typical ExperimentsDocument5 pagesColorimeter Sensor DT185A: Typical ExperimentscarmenamorariteiNo ratings yet
- DR 2800 Spectrophotometer Procedures ManualDocument814 pagesDR 2800 Spectrophotometer Procedures Manualzvjesos100% (3)
- New Astm d7066-04Document4 pagesNew Astm d7066-04Aris RahmanNo ratings yet
- Technical Data Sheet: Chemeon TCP-HF™Document8 pagesTechnical Data Sheet: Chemeon TCP-HF™vijay yadavNo ratings yet