Professional Documents
Culture Documents
Chem 280 Mock Exam Week 1 Withkey - 1 - 1
Uploaded by
Gricelda Vasquez0 ratings0% found this document useful (0 votes)
87 views3 pagesThis document contains a 16 question chemistry test with multiple choice and calculation questions. It instructs students to show all work for numerical problems and that no work shown results in no credit. It also provides spaces for students to write their answers to the questions.
Original Description:
Original Title
Chem 280 Mock exam week 1 withkey_1_ _1_ (1)
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document contains a 16 question chemistry test with multiple choice and calculation questions. It instructs students to show all work for numerical problems and that no work shown results in no credit. It also provides spaces for students to write their answers to the questions.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
87 views3 pagesChem 280 Mock Exam Week 1 Withkey - 1 - 1
Uploaded by
Gricelda VasquezThis document contains a 16 question chemistry test with multiple choice and calculation questions. It instructs students to show all work for numerical problems and that no work shown results in no credit. It also provides spaces for students to write their answers to the questions.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 3
Name: ___________________________________
For problems or questions requiring a numerical answer, show all
calculation set-ups. No work = No credit!
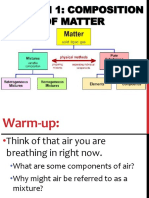
1. Which of the following is not a pure substance? (1 pt.)
a) nitrogen gas c) iron
b) air d) sodium chloride
2. An example of a chemical property of formaldehyde (CH2O) is
(1 pt.)
a) It is a colorless gas.
b) It has a density of 0.815 g/ml.
c) It is corrosive.
d) It dissolves in alcohol.
3. Which of the following is a physical change? (1 pt.)
a) An iron nail rusts.
b) Mixing vinegar and solid baking soda produces bubbles.
c) Dry ice sublimes at room temperature.
d) Methane gas combusts in the presence of oxygen gas.
4. Which of the following terms is most appropriate when classi-
fying a pepperoni pizza? (1 pt.)
a) pure substance c) heterogeneous mixture
b) compound d) homogeneous mixture
5. Saltpeter is a mineral found in many parts of the world. A
sample of saltpeter taken from anywhere in the world has a
composition 38.7% K, 13.9% N, and 47.4% O. Based on this,
it can be concluded that saltpeter is (1 pt.)
a) an element. c) a heterogeneous mixture.
b) a compound. d) a homogenous mixture.
6. How many significant figures are in each of the following
numbers? (4 pts.)
a) 3.1400 _5___ c) 0.0006042 _4___
b) 45,300 __Ambiguous__ d) 0.04560700 7____
7. A flask contains 145.675 ml of saline solution. If 24.2 ml of
the saline solution are withdrawn from the flask, how should
the volume of the saline solution that remains in the flask
be reported (to the proper number of significant figures)?
(1 pt.)
a) 121.475 ml d) 121 ml
b) 121.48 ml e) 122 ml
c) 121.5 ml
8. Carry out the following calculation and report the answer
using the proper number of significant figures: (1 pt.)
a) 31.0185 ft/s d) 31.0 ft/s
b) 31.01 ft/s e) 31 ft/s
c) 31.02 ft/s
9. A typical red blood cell has a diameter of 7.50 x 10-6 m.
What is the diameter of a typical red blood cell in mm?
(2 pts.)
7.50x10-3 mm
10. How many liters are contained in a 67.6 ounce soft-drink bot-
tle? (1.00 ounce = 29.6 ml) (3 pts.)
2.00L
11. How many miles are covered in a 15 km race? (1 mile = 5280
feet, 12 inches = 1 foot, 1 inch = 2.54 cm) (5 pts.)
93. miles
12. If a 185-lb patient is prescribed 145 mg of the cholesterol-
lowering drug Tricor daily, what dosage is the patient re-
ceiving in mg/kg of body mass? (1 pound = 454 grams) (3 pts.)
173 mg/Kg
13. 1.5 L of normal saline solution is required to be given over
4 hours. If the IV at the hospital delivers 10 drops per ml,
calculate the drip rate-in drops per minute-that the patient
needs to be given. (4 pts.)
62.5 drops/min
14. Ray Bradbury chose Fahrenheit 451 as the title of his novel
about book burning in the future because that’s the tempera-
ture at which paper ignites. What would his title been in
degrees Celsius and in Kelvin? (2 pts.)
2330C
15. An irregularly shaped object has a mass of 66.12 grams. When
it is placed in a graduated cylinder containing 12.5 ml of
water, the water level rises to 22.7 ml. Calculate the den-
sity of this object. (3 pts.)
6.48 g/mL
16. Record the volume of water in the graduated cylinder with the
proper number of significant figures. (1 pt.) ____12.8 mL____
You might also like
- Chapter 3 AssessmentDocument18 pagesChapter 3 AssessmentRuel Cedeño100% (3)
- Chapter 01 - Chemistry and MeasurementDocument56 pagesChapter 01 - Chemistry and MeasurementJamie B100% (1)
- O Level Biology Practice Questions And Answers EnzymesFrom EverandO Level Biology Practice Questions And Answers EnzymesRating: 5 out of 5 stars5/5 (1)
- Surviving Chemistry: A Guided Study Book For High School ChemistryDocument83 pagesSurviving Chemistry: A Guided Study Book For High School ChemistryE3 Scholastic Publishing100% (2)
- General Chemistry I - Tutorial 1Document7 pagesGeneral Chemistry I - Tutorial 1Duc Anh NguyenNo ratings yet
- BT HPTDocument31 pagesBT HPTLinh NguyenNo ratings yet
- Practice Exam AnswersDocument19 pagesPractice Exam AnswersNguyễn Minh AnhNo ratings yet
- Before The Exam BeginsDocument7 pagesBefore The Exam BeginsH!T0% (1)
- Science: Quarter 1 - Module 1-MixtureDocument24 pagesScience: Quarter 1 - Module 1-MixtureJerome Manaig SueltoNo ratings yet
- Chem 280 Mock Exam Week 1 Withkey - 1 - 1Document3 pagesChem 280 Mock Exam Week 1 Withkey - 1 - 1Gricelda VasquezNo ratings yet
- A Detailed Lesson Plan in ScienceDocument4 pagesA Detailed Lesson Plan in SciencemarigoldNo ratings yet
- Tutorial 1 General ChemistryDocument5 pagesTutorial 1 General ChemistryFrost OrchidNo ratings yet
- General Chemistry Tutorial 1 ConceptsDocument5 pagesGeneral Chemistry Tutorial 1 ConceptsKedai KasutNo ratings yet
- AP Mid Term ReviewDocument44 pagesAP Mid Term Reviewmetalover36No ratings yet
- Chem Questions and Answers 151 FinalDocument12 pagesChem Questions and Answers 151 FinalTom TeslaNo ratings yet
- Quiz 1 - GadiDocument3 pagesQuiz 1 - GadiJerome GadiNo ratings yet
- Mole Concept Confidence Building Test1Document12 pagesMole Concept Confidence Building Test11harshikaNo ratings yet
- General Chemistry I - Tutorial 1Document5 pagesGeneral Chemistry I - Tutorial 1Khuê Nguyễn ThếNo ratings yet
- Sample QuestionsDocument20 pagesSample QuestionsginizyNo ratings yet
- Chapter 12 HomeworkDocument6 pagesChapter 12 HomeworkdmanjdpNo ratings yet
- St00502 Basic Chemistry Assignment 1 Answer All of The QuestionsDocument2 pagesSt00502 Basic Chemistry Assignment 1 Answer All of The QuestionsOri LukeNo ratings yet
- Chapter-7 Solution-Properties ExercisesDocument13 pagesChapter-7 Solution-Properties Exercisestran huyNo ratings yet
- General, Organic, and Biological Chemistry Practice Exam QuestionsDocument24 pagesGeneral, Organic, and Biological Chemistry Practice Exam QuestionsAdham AhmedNo ratings yet
- Chemistry Lab Exam PDFDocument10 pagesChemistry Lab Exam PDFnitinNo ratings yet
- Chemistry 192 Calculation WorksheetDocument3 pagesChemistry 192 Calculation WorksheetLeah Cortel TesorioNo ratings yet
- DPP 1Document10 pagesDPP 1Phani PadmasriNo ratings yet
- Chapter 13Document4 pagesChapter 13Poonam CheemaNo ratings yet
- Sample (X) Sample ExamDocument4 pagesSample (X) Sample ExamLaia ValenciaNo ratings yet
- HW #2 - CH 16, 17, 19Document6 pagesHW #2 - CH 16, 17, 19Ingrid IsabelNo ratings yet
- Chapter 1 WorksheetDocument5 pagesChapter 1 WorksheetJules BrunoNo ratings yet
- Soal-Soal KimiaDocument6 pagesSoal-Soal KimiasaripurwantiNo ratings yet
- USTP College of Science and Mathematics Midterm AssignmentDocument2 pagesUSTP College of Science and Mathematics Midterm AssignmentCarl Harvey OcionesNo ratings yet
- Microsoft Word - Tutorial 1 CLB 10004Document4 pagesMicrosoft Word - Tutorial 1 CLB 10004wanizalilNo ratings yet
- Pharma TechnologyDocument8 pagesPharma TechnologymuhammadNo ratings yet
- CHM100 Practice Exam 2Document8 pagesCHM100 Practice Exam 2Abdullah AltwirqiNo ratings yet
- CHEM 113-Quiz #7 Answer KeyDocument4 pagesCHEM 113-Quiz #7 Answer KeySteve RiddlerNo ratings yet
- Exam 4b Chem 120 Spring 2013Document7 pagesExam 4b Chem 120 Spring 2013ASEELNo ratings yet
- 2019leqtr3 g11 Stem Chem1Document13 pages2019leqtr3 g11 Stem Chem1Elcid BocacaoNo ratings yet
- Chapter 1Document22 pagesChapter 1StephenNo ratings yet
- Quiz BeeDocument85 pagesQuiz BeeFierre NouxNo ratings yet
- Black HoleDocument2 pagesBlack HoleLouis Fetilo Fabunan0% (1)
- Edexcel GCE Chemistry Unit-4 June 2014 Question Paper (R)Document24 pagesEdexcel GCE Chemistry Unit-4 June 2014 Question Paper (R)AvrinoxNo ratings yet
- Tutorial DACS1232 AllDocument6 pagesTutorial DACS1232 Allluqman04hakimiNo ratings yet
- Fundamentals of General Organic and Biological Chemistry With MasteringChemistry 7th Edition McMurry Test Bank 1Document14 pagesFundamentals of General Organic and Biological Chemistry With MasteringChemistry 7th Edition McMurry Test Bank 1carmen100% (36)
- General Chemistry 1 Diagnostic Exam STEM 12Document11 pagesGeneral Chemistry 1 Diagnostic Exam STEM 12Neil CorveraNo ratings yet
- Apch01 pt02Document3 pagesApch01 pt02dheerajkumarsahNo ratings yet
- CH 1. Matter and MeasurementDocument10 pagesCH 1. Matter and Measurementewewwe weweweweNo ratings yet
- Chapter 5 GasesDocument37 pagesChapter 5 GasesNeally WeallyNo ratings yet
- Review 1 LinkedDocument3 pagesReview 1 LinkedBurny BurnerNo ratings yet
- Che 1210 Tutorial Sheet 1 2024Document2 pagesChe 1210 Tutorial Sheet 1 2024simawuchristine100% (1)
- 1) (15 PTS) A 10 Inch Diameter Sanitary Sewer Is Designed Such That ItDocument11 pages1) (15 PTS) A 10 Inch Diameter Sanitary Sewer Is Designed Such That ItMukter HossainNo ratings yet
- Test 2 Version-3 Print Version-22-12-With KeyDocument4 pagesTest 2 Version-3 Print Version-22-12-With KeymNo ratings yet
- Revision 2Document10 pagesRevision 2LSWNo ratings yet
- Honors Chemistry ReviewDocument7 pagesHonors Chemistry ReviewngctynNo ratings yet
- Chem WorksheetDocument1 pageChem WorksheetAngelyn RabajaNo ratings yet
- Chemistry CT 2 22-23Document6 pagesChemistry CT 2 22-23Sancia SamNo ratings yet
- PharmacyDocument3 pagesPharmacykarimakki100% (1)
- CHM 1025c Final Exam Practice WCDocument5 pagesCHM 1025c Final Exam Practice WCMiguel SuarezNo ratings yet
- 9th Science Dav Sa-1 Question PaperDocument7 pages9th Science Dav Sa-1 Question PapermukulNo ratings yet
- Ranjeet Shahi Chemistry PapersDocument10 pagesRanjeet Shahi Chemistry Paperssabhari_ram100% (1)
- Full Download Test Bank For Principles of General Chemistry 3rd Edition Martin Silberberg PDF Full ChapterDocument36 pagesFull Download Test Bank For Principles of General Chemistry 3rd Edition Martin Silberberg PDF Full Chaptershraggerdisprovet6dv100% (17)
- Basic Concepts in ChemistryDocument2 pagesBasic Concepts in ChemistryMohamed Yahia100% (2)
- Lab CH 32Document6 pagesLab CH 32Gricelda VasquezNo ratings yet
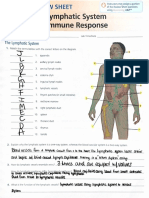
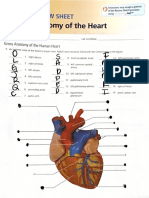
- Nasal cavities and respiratory tract anatomyDocument4 pagesNasal cavities and respiratory tract anatomyGricelda VasquezNo ratings yet
- Lab CH35Document3 pagesLab CH35Gricelda VasquezNo ratings yet
- Lab CH 30Document4 pagesLab CH 30Gricelda VasquezNo ratings yet
- Lab CH 27Document4 pagesLab CH 27Gricelda VasquezNo ratings yet
- Lab CH 26Document2 pagesLab CH 26Gricelda VasquezNo ratings yet
- Lab CH 23Document4 pagesLab CH 23Gricelda VasquezNo ratings yet
- Lab CH 17Document6 pagesLab CH 17Gricelda VasquezNo ratings yet
- Lab CH 25Document4 pagesLab CH 25Gricelda VasquezNo ratings yet
- Lab CH 19Document3 pagesLab CH 19Gricelda VasquezNo ratings yet
- Lab CH 15 PDFDocument4 pagesLab CH 15 PDFGricelda VasquezNo ratings yet
- Lab CH 15 PDFDocument4 pagesLab CH 15 PDFGricelda VasquezNo ratings yet
- Preparing Mixtures: Homogeneous vs HeterogeneousDocument2 pagesPreparing Mixtures: Homogeneous vs HeterogeneousCarina VillalobosNo ratings yet
- Science6 1 Quarter Practice Test Fill in The Blanks:: DecantationDocument4 pagesScience6 1 Quarter Practice Test Fill in The Blanks:: DecantationPinky LaysaNo ratings yet
- 01-02 Heterogeneous Homogeneous and Pure Worksheet - AnswersDocument1 page01-02 Heterogeneous Homogeneous and Pure Worksheet - AnswersNOVA LESLIE AGAPAYNo ratings yet
- Lesson Plan No 1Document4 pagesLesson Plan No 1Royeni KurniawatiNo ratings yet
- Grade 9 Science Exam ReviewDocument3 pagesGrade 9 Science Exam ReviewNeptune LopezNo ratings yet
- Science Class 9 PDFDocument228 pagesScience Class 9 PDFVansh BhardwajNo ratings yet
- Is Matter Around Us PureDocument46 pagesIs Matter Around Us Pureparamjeet164No ratings yet
- Is Matter Around Us PureDocument23 pagesIs Matter Around Us PureAryan AgarwalNo ratings yet
- Class 9 Matter Around You Is PureDocument15 pagesClass 9 Matter Around You Is PurebrcraoNo ratings yet
- 1.4 Pure Substances Mixtures PPTDocument42 pages1.4 Pure Substances Mixtures PPTFebbie YanNo ratings yet
- MixturesDocument5 pagesMixturesLøwkeÿÿ MøntanaNo ratings yet
- Composition and Properties of MatterDocument23 pagesComposition and Properties of MatterLynnel BoterNo ratings yet
- Diagnostic Exam Hau Geas - Key PDFDocument4 pagesDiagnostic Exam Hau Geas - Key PDFMananquil JeromeNo ratings yet
- Substances and Mixtures: How Are Mixtures Different From Substances? How Are They Similar?Document10 pagesSubstances and Mixtures: How Are Mixtures Different From Substances? How Are They Similar?Alex Buzarang SubradoNo ratings yet
- Chemistry: Classifying MatterDocument3 pagesChemistry: Classifying MatterMa. Filipina AlejoNo ratings yet
- Physical and Chemical Changes QuizDocument8 pagesPhysical and Chemical Changes Quizkjj7760No ratings yet
- 2.5 Substances and MixturesDocument15 pages2.5 Substances and MixturesNora PasaNo ratings yet
- Classifying Matter and Energy StatesDocument7 pagesClassifying Matter and Energy StatesJam Uly GastyNo ratings yet
- MELCs for Materials and MixturesDocument2 pagesMELCs for Materials and MixturesSARAH FABIANNo ratings yet
- Matter and Its PropertiesDocument45 pagesMatter and Its PropertiesLu NaNo ratings yet
- Is Matter Around Us PureDocument14 pagesIs Matter Around Us Purepunksnotdead_t7100% (1)
- Mysterious Mixtures ExperimentDocument13 pagesMysterious Mixtures ExperimentJerneth Nyka FloresNo ratings yet
- WWW Askiitians Com Revision Notes Class 9 Science Is Matter Around Us PureDocument17 pagesWWW Askiitians Com Revision Notes Class 9 Science Is Matter Around Us PureDeepak Kumar SharmaNo ratings yet
- Physical and Chemical Properties of MatterDocument75 pagesPhysical and Chemical Properties of MatterKevin TranNo ratings yet