Professional Documents
Culture Documents
A. F (Fluorine) B. Ba (Barium) C. FR (Francium) D. Si (Silicon) E. NP (Neptunium) F. Mo (Molybdenum) G. Og (Oganesson) H. He (Helium)
Uploaded by
peter vanderOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
A. F (Fluorine) B. Ba (Barium) C. FR (Francium) D. Si (Silicon) E. NP (Neptunium) F. Mo (Molybdenum) G. Og (Oganesson) H. He (Helium)
Uploaded by
peter vanderCopyright:
Available Formats
PETER A.
VANDER ZANDER BETET-NS 1A
Practice Exercise 3.5
A. F (Fluorine)
B. Ba (Barium)
C. Fr (francium)
D. Si (Silicon)
E. Np (Neptunium)
F. Mo (Molybdenum)
G. Og (Oganesson)
H. He (Helium)
1. the most metallic element= C.) Fr (francium)
2. the most non-metallic element= H.) He (Helium)
3. The element with the biggest atomic size= C.) Fr (francium)
4. The element classified as alkali metal= C.) Fr (francium)
5. The element classified as metalloid= D.) Si (Silicon)
6. The element classified as alkaline-earth metal= B.) Ba (Barium)
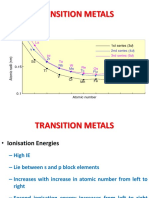
7. The transition element= F.) Mo (Molybdenum)
8. the element classified as halogen= A.) F (Fluorine)
9. The lightest of the noble gases= H.) He (Helium)
10. Element with electronic configuration ending in f= E.) Np (Neptunium)
11. Element with four (4) valence electrons= D.) Si (Silicon)
12-Element with electronic configuration ending in d= A.) F (Fluorine)
13. Element with seven (7) valence electrons= B.) Ba (Barium)
14. Element with eight valence electrons= G.) Og (Oganesson)
15. Element with one occupied main energy level= H.) He (Hel
Symbol Atomic Abbreviated Group No. of Electron Period Number LSC classification
No. Electronic No. Valence dot No. of
configuration Electron formula occupied
m.e.l
Alkalai
K (Potassium) 19 [ Ar] 4s1 1A 1 e- 4 4 4s2 metals
Alkaline
Mg 12 [Ne] 3s2 2A 2 e- 3 3 3s2 earth metal
(Magnesium)
Carbon
Ge (Germanium 32 [Ar] 4s2,4p2 4A 4e- 4 4 4p2 family
Alkalai
In (Indium) 49 [kr] 5p1 1A 1e- 5 5 5p1 metals
Nitrogen
Ta (Tantalum) 73 [Xe] 5d3,6s2 5A 5 e- 6 6 6s2 family
Alkaline
Ti (Titanium) 22 [Ar] 3d2 2A 2 e- 3 4 3d2 earth metal
Noble gas
6 - 6
Rn (Radon) 86 [xe] 6p 6A 6e 6 6 6p
You might also like
- Blues CheatDocument77 pagesBlues CheatRobbie ChambersNo ratings yet
- Lab 3 Atomic Structure (Chem 136)Document5 pagesLab 3 Atomic Structure (Chem 136)NatNo ratings yet
- The Georgian Technical University The Department of Electrical Engineering and Electronics Simon NemsadzeDocument231 pagesThe Georgian Technical University The Department of Electrical Engineering and Electronics Simon Nemsadzepeter vanderNo ratings yet
- The Entheogen Review׃ Vol. 16, No. 1 (2008)Document44 pagesThe Entheogen Review׃ Vol. 16, No. 1 (2008)HoorayFrisbeeHead100% (2)
- PESTEL Analysis of MoroccoDocument2 pagesPESTEL Analysis of Moroccoethernalx67% (15)
- 1270A544-032 Console v3.1Document304 pages1270A544-032 Console v3.1badr eddine100% (1)
- Sky & Telescope - February 2016 (Gnv64)Document82 pagesSky & Telescope - February 2016 (Gnv64)bogarguz100% (1)
- Fire Alarm Detection System With Short Service Message (SMS) TechnologyDocument29 pagesFire Alarm Detection System With Short Service Message (SMS) Technologypeter vander100% (1)
- Rod and Pump DataDocument11 pagesRod and Pump DataYoandri Stefania Guerrero CamargoNo ratings yet
- Bombardier Zefiro Technical Description enDocument15 pagesBombardier Zefiro Technical Description ennickerlesstezla100% (1)
- OVERVIEWDocument16 pagesOVERVIEWjae eNo ratings yet
- Periodic TableDocument2 pagesPeriodic TablePeter Vander ZanderNo ratings yet
- Chemical Elements: Metals Metalloids NonmetalsDocument7 pagesChemical Elements: Metals Metalloids NonmetalsNavya NarayanNo ratings yet
- 5-d&f Block ElementsDocument207 pages5-d&f Block ElementsArkaNo ratings yet
- JEE D and F Block Notes Final CompressedDocument207 pagesJEE D and F Block Notes Final CompressedaeroenthusiastaltaltNo ratings yet
- First Transition Series: D and F - Block Elements IntroductionDocument4 pagesFirst Transition Series: D and F - Block Elements IntroductionAbdul QayyumNo ratings yet
- Alkaline Earth Metal - WikipediaDocument29 pagesAlkaline Earth Metal - WikipediaOladimeji OluwakemiNo ratings yet
- D and F Block 09 08 2022Document14 pagesD and F Block 09 08 2022Prajwal SrinathNo ratings yet
- CH 15 PDFDocument64 pagesCH 15 PDFkrishnaNo ratings yet
- 5.3.2 Transition Metals PDFDocument11 pages5.3.2 Transition Metals PDFkrishnaviNo ratings yet
- CH 14 PDFDocument26 pagesCH 14 PDFkrishnaNo ratings yet
- Notes On Coordination CompoundsDocument12 pagesNotes On Coordination CompoundsRojo JohnNo ratings yet
- Inorganic Reaction Mechanisms: January 2020Document225 pagesInorganic Reaction Mechanisms: January 2020AdistaNo ratings yet
- Transition Elements (B.sc-Ii) Inorganic Chemistry Paper-IDocument32 pagesTransition Elements (B.sc-Ii) Inorganic Chemistry Paper-IPinky SinghNo ratings yet
- All About The Periodic Table - Home Laboratory WorksheetDocument4 pagesAll About The Periodic Table - Home Laboratory WorksheetFrank Ed SerranoNo ratings yet
- M.D.Sangale, A.S.Daptare, N.G.Shinde.: AbstractDocument7 pagesM.D.Sangale, A.S.Daptare, N.G.Shinde.: AbstractJohan Raj VerdiansyahNo ratings yet
- Crystal Field Theory - 4 Marks in 10 Minutes For NEET ExamDocument3 pagesCrystal Field Theory - 4 Marks in 10 Minutes For NEET Exammesati6896No ratings yet
- Chemistry 12 CH06NotesDocument33 pagesChemistry 12 CH06NotesKhalidsaifullahNo ratings yet
- CourseDocument9 pagesCourseflamepixerxNo ratings yet
- Class 12 CH 9 Coordination CompoundsDocument4 pagesClass 12 CH 9 Coordination CompoundsananthusbNo ratings yet
- Chapter 8 Lecture NotesDocument10 pagesChapter 8 Lecture NotesRayan BaigNo ratings yet
- OrangometaicDocument9 pagesOrangometaicscimohammedkamalfathyabudeifNo ratings yet
- Alkali Metal - WikipediaDocument6 pagesAlkali Metal - WikipediaSasuka UchewaNo ratings yet
- Transition Metals 2021Document49 pagesTransition Metals 2021Jomy Jose PhilipNo ratings yet
- Block Elements Class 12Document15 pagesBlock Elements Class 12Åmìßhã PŕãťãpNo ratings yet
- Review Article: Rare Earth Elements: Their Importance in Understanding Soil GenesisDocument12 pagesReview Article: Rare Earth Elements: Their Importance in Understanding Soil Genesissphericity 2018No ratings yet
- Classifying Periodic Table of Elements-ActiviDocument1 pageClassifying Periodic Table of Elements-ActiviKimberly Joy LungayNo ratings yet
- Clusters: Coordination ChemistryDocument4 pagesClusters: Coordination ChemistryBadalNo ratings yet
- Coordination Compounds 2022Document16 pagesCoordination Compounds 2022SIDHARTH SINHNo ratings yet
- Elementeve Kimike Z Emri: Tabela e Seria Kimike (G/mol)Document9 pagesElementeve Kimike Z Emri: Tabela e Seria Kimike (G/mol)AaaaNo ratings yet
- 6529242dd6ffef0018ccb77e - ## - P - Block (Part - 01)Document32 pages6529242dd6ffef0018ccb77e - ## - P - Block (Part - 01)justrohithingsNo ratings yet
- Coordination ChemistryDocument30 pagesCoordination ChemistryRizwanbhatNo ratings yet
- Lecture Notes PDFDocument60 pagesLecture Notes PDFprakas.rao39695No ratings yet
- Chemical Properties of Amphibole Supergroup: Cannilloite Ferri-Cannilloite Ferri-Fluoro-CannilloiteDocument1 pageChemical Properties of Amphibole Supergroup: Cannilloite Ferri-Cannilloite Ferri-Fluoro-CannilloitenetsecureNo ratings yet
- Werner's Coordination TheoryDocument3 pagesWerner's Coordination Theoryasole23No ratings yet
- Lilavatibai Podar High School (Isc) : Holding Capacity of Shells: 2 N Formula (N Position of The Shell From The NucleusDocument3 pagesLilavatibai Podar High School (Isc) : Holding Capacity of Shells: 2 N Formula (N Position of The Shell From The NucleusMahesh hamneNo ratings yet
- Coordination ChemistryDocument76 pagesCoordination ChemistryLipsa PradhanNo ratings yet
- KryptonDocument4 pagesKryptonJarky SparcsNo ratings yet
- Periodic TableDocument169 pagesPeriodic TableMalik DaniyalNo ratings yet
- 6 P Block Elements PDFDocument91 pages6 P Block Elements PDFShanmugapriya RaguramanNo ratings yet
- BMPeriodic TableDocument2 pagesBMPeriodic TablehoneyoopsyaaNo ratings yet
- Coordination CompoundsDocument51 pagesCoordination CompoundsasdfNo ratings yet
- Chemistry Worksheet 4Document3 pagesChemistry Worksheet 4Brian Laurence BarroNo ratings yet
- Chapter 5 Coordination CompoundDocument36 pagesChapter 5 Coordination Compoundammar zakariaNo ratings yet
- 바닥상태 전자배치와 원자가전자Document3 pages바닥상태 전자배치와 원자가전자a01042932313No ratings yet
- Solution 2Document12 pagesSolution 2Varad DNo ratings yet
- Anomalies On Electron ConfigurationsDocument1 pageAnomalies On Electron ConfigurationsKathleen PiedadNo ratings yet
- Perodic Table Groups: ValencyDocument2 pagesPerodic Table Groups: ValencykckskdnakNo ratings yet
- Ionic Radius - Wikipedia PDFDocument29 pagesIonic Radius - Wikipedia PDFடேவிட் ஸ்No ratings yet
- List of Important Metals and Their Ores With Chemical Formulas PDFDocument2 pagesList of Important Metals and Their Ores With Chemical Formulas PDFAudibleNo ratings yet
- P2S Chemistry The D & F Block Elements, Coordination CompoundsDocument108 pagesP2S Chemistry The D & F Block Elements, Coordination Compoundsavith777No ratings yet
- Coordination Chemistry: Complex IonDocument11 pagesCoordination Chemistry: Complex Ionserdia muhammadNo ratings yet
- Periodic Table of The Elements: Li Na K RB CsDocument1 pagePeriodic Table of The Elements: Li Na K RB CsLukeThorburnNo ratings yet
- Coordination Chemistry:: An OverviewDocument38 pagesCoordination Chemistry:: An OverviewAnmol KalantriNo ratings yet
- 5 Transition Metals Set 2Document3 pages5 Transition Metals Set 2Thinaya JayarathneNo ratings yet
- Elias Inorg Lec 5 PDFDocument14 pagesElias Inorg Lec 5 PDFNidhi SisodiaNo ratings yet
- 18 Electron Rule: How To Count ElectronsDocument14 pages18 Electron Rule: How To Count ElectronsGA GANo ratings yet
- Xii-Chem-Chptr-3-S-Block ElementsDocument12 pagesXii-Chem-Chptr-3-S-Block ElementsTanveer AhmedNo ratings yet
- The Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyFrom EverandThe Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyNo ratings yet
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972From EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverNo ratings yet
- Activity No. 5 - Electrical Conductivity ofDocument5 pagesActivity No. 5 - Electrical Conductivity ofpeter vanderNo ratings yet
- E-Learning Management System For Thesis Process Support From A Supervisor PerspectiveDocument75 pagesE-Learning Management System For Thesis Process Support From A Supervisor Perspectivepeter vanderNo ratings yet
- Activity No. 2 - Common Laboratory Techiques - VanderzanderDocument2 pagesActivity No. 2 - Common Laboratory Techiques - Vanderzanderpeter vanderNo ratings yet
- Assignment No.1 PATULAYDocument7 pagesAssignment No.1 PATULAYpeter vanderNo ratings yet
- Frequency, Period and Time: Electrical Engineering Technology DepartmentDocument2 pagesFrequency, Period and Time: Electrical Engineering Technology Departmentpeter vanderNo ratings yet
- Module 2 - VanderzanderDocument6 pagesModule 2 - Vanderzanderpeter vanderNo ratings yet
- The Equation of A Sinusoidal Waveform: Electrical Engineering Technology DepartmentDocument3 pagesThe Equation of A Sinusoidal Waveform: Electrical Engineering Technology Departmentpeter vanderNo ratings yet
- Module 3 VANDERZANDERDocument4 pagesModule 3 VANDERZANDERpeter vanderNo ratings yet
- Paulines Plate 6 PDFDocument93 pagesPaulines Plate 6 PDFpeter vanderNo ratings yet
- Final Lab Report 2 DataDocument17 pagesFinal Lab Report 2 Datapeter vanderNo ratings yet
- Worksheet 1 Community Mapping PATULAYDocument3 pagesWorksheet 1 Community Mapping PATULAYpeter vanderNo ratings yet
- Weekly Home Learning Plan - KindergartenDocument3 pagesWeekly Home Learning Plan - KindergartenPrecious ArniNo ratings yet
- QP English Viii 201920Document14 pagesQP English Viii 201920Srijan ChaudharyNo ratings yet
- General Office Administration Level 1 (CVQ) PDFDocument129 pagesGeneral Office Administration Level 1 (CVQ) PDFddmarshall2838No ratings yet
- Kitimat JRP SummaryDocument17 pagesKitimat JRP SummaryNorthwest InstituteNo ratings yet
- Service Oriented Architecture - Course Notes PDFDocument93 pagesService Oriented Architecture - Course Notes PDFRoberto Luna OsorioNo ratings yet
- Verilog HDL: Special ClassesDocument11 pagesVerilog HDL: Special ClassesUnique ProNo ratings yet
- Mathematics IDocument247 pagesMathematics IShreya PankajNo ratings yet
- Recognition SpielDocument4 pagesRecognition SpielJoeyNo ratings yet
- NN IP Guidebook To Alternative CreditDocument140 pagesNN IP Guidebook To Alternative CreditBernardNo ratings yet
- Introduction To Public Policy: Course DescriptionDocument15 pagesIntroduction To Public Policy: Course DescriptionJericko Perez AvilaNo ratings yet
- Projects & Operations: IN: NE Power Systm ImprvmDocument5 pagesProjects & Operations: IN: NE Power Systm ImprvmGaurang PatelNo ratings yet
- Appraisal Manual For TeachersDocument16 pagesAppraisal Manual For TeachersCleveLeungNo ratings yet
- James Rachels - What Is MoralityDocument26 pagesJames Rachels - What Is MoralityKiara Lagrisola100% (1)
- Formalismo Geométrico de La Mecánica Cuántica y Sus Aplicaciones A Modelos MolecularesDocument51 pagesFormalismo Geométrico de La Mecánica Cuántica y Sus Aplicaciones A Modelos Moleculareshugo_valles_2No ratings yet
- Genre and SubgenreDocument15 pagesGenre and SubgenreMauricio GalvezNo ratings yet
- QP-Computer Science-12-Practice Paper-1Document8 pagesQP-Computer Science-12-Practice Paper-1subashreebaski16No ratings yet
- Node Name Ping Status Community IP Address: CAPSTONE - IT Infrastructure Monitoring SNMP WalkDocument4 pagesNode Name Ping Status Community IP Address: CAPSTONE - IT Infrastructure Monitoring SNMP WalkSpartacus CaesarNo ratings yet
- L807268EDocument1 pageL807268EsjsshipNo ratings yet
- Cept FinalDocument14 pagesCept FinalVighnesh MalagiNo ratings yet
- (R) Nelson JB (2017) - Mindful Eating - The Art of Presence While You EatDocument4 pages(R) Nelson JB (2017) - Mindful Eating - The Art of Presence While You EatAnonymous CuPAgQQNo ratings yet
- 805-Article Text-3656-1-10-20220310Document16 pages805-Article Text-3656-1-10-20220310abolfazlshamsNo ratings yet
- Drug-Induced Sleep Endoscopy (DISE)Document4 pagesDrug-Induced Sleep Endoscopy (DISE)Luis De jesus SolanoNo ratings yet