Professional Documents
Culture Documents
Solving the Van Der Waals Equation for Molar Volume and Compressibility Factor
Uploaded by
jhon smithOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Solving the Van Der Waals Equation for Molar Volume and Compressibility Factor
Uploaded by
jhon smithCopyright:
Available Formats
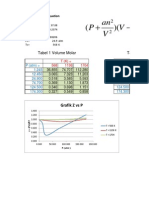
Problem 1.
1 Molar Volume and Compressibility factor from Van Der Waals Equation
Part a - Solution for P=56 (atm) and T = 450 K
Equations Initial val Solution1
V 0.574892 0.7 0.574892
P = 56 56 56 56
R = 0.08206 0.08206 0.08206 0.08206
T = 450 450 450 450
Tc = 405.5 405.5 405.5 405.5
Pc = 111.3 111.3 111.3 111.3
Pr = P/Pc 0.503145 0.503145 0.503145
a = 27*(R^2*T 4.196946 4.196946 4.196946
b = R*Tc/(8*Pc 0.037371 0.037371 0.037371
Z = P*V/(R*T) 0.871827 1.061554 0.871827
f(V) = (P+a/(V^ 8.5E-07 5.855757 8.5E-07
1
Solution is obtained by Goal Seek(see under Tools dropdown menu) by setting the value
of the cell b14 at zero while changing cell b4
You might also like
- Resultados Con PolymathDocument11 pagesResultados Con PolymathMiguel CapuñayNo ratings yet
- Excel Solutions To The Chemical Engineering Problem SetDocument59 pagesExcel Solutions To The Chemical Engineering Problem SetSakura NuguishiNo ratings yet
- Tut 4 VLE of Pure Fluids - SolutionsDocument13 pagesTut 4 VLE of Pure Fluids - SolutionsAsma NasserNo ratings yet
- Solving Basic Problems Using Spreadsheets RIVERAL CHOYSON S.Document5 pagesSolving Basic Problems Using Spreadsheets RIVERAL CHOYSON S.CHOYSON RIVERALNo ratings yet
- Calculate bubble point temperature and dew point pressure using Antoine equationDocument4 pagesCalculate bubble point temperature and dew point pressure using Antoine equationCamilla Mae LibunaoNo ratings yet
- An P V NB NRT V: Tabel 1 Volume Molar Tabel 2 Z PV/RTDocument3 pagesAn P V NB NRT V: Tabel 1 Volume Molar Tabel 2 Z PV/RTDitya RistiantiNo ratings yet
- CHEG 201 Chemical Process Calculation HomeworkDocument11 pagesCHEG 201 Chemical Process Calculation HomeworkAASHISH CHAULAGAINNo ratings yet
- 11 Basic Principles and CalculationsDocument64 pages11 Basic Principles and CalculationsJue RasepNo ratings yet
- Khusus Trial Deodoriser BenerDocument14 pagesKhusus Trial Deodoriser BenerRachmad Darmawan EnsaNo ratings yet
- Ship Propulsion Problems and SolutionsDocument20 pagesShip Propulsion Problems and SolutionsYe Wint ThuNo ratings yet
- Workbook Workbook Workbook Workbook Workbook: Try Yourself QuestionsDocument14 pagesWorkbook Workbook Workbook Workbook Workbook: Try Yourself QuestionsarunNo ratings yet
- EE201 Circuit Transient Problems Nov07Document5 pagesEE201 Circuit Transient Problems Nov07Siddaraj UppinNo ratings yet
- Tryout Exam - SolutionDocument8 pagesTryout Exam - SolutionZakariya MohamedNo ratings yet
- Tugas 2 Thermodynamics 2 (Gayuh Aditya Hutomo 5213419053)Document4 pagesTugas 2 Thermodynamics 2 (Gayuh Aditya Hutomo 5213419053)Gayuh Aditya HutomoNo ratings yet
- SOLUTION-Introduction To Modern Power ElectronicsDocument37 pagesSOLUTION-Introduction To Modern Power Electronicsluckywanker33% (3)
- Ecuaciones Cubicas: Van Der WallsDocument7 pagesEcuaciones Cubicas: Van Der WallsMiguelAngelAntezanaVergaraNo ratings yet
- Full-Wave Controlled Rectifier RL Load (Continuous Mode)Document6 pagesFull-Wave Controlled Rectifier RL Load (Continuous Mode)hamza abdo mohamoud100% (1)
- Dayeuh ManggungDocument1 pageDayeuh ManggungRifa MiladNo ratings yet
- Termo DaningDocument20 pagesTermo DaningRahasia Tahu RahasiaNo ratings yet
- Distillation: Abdulsalam Sufyaan Hamed Mustafa Jumaah Abbas Zahraa ShehabDocument17 pagesDistillation: Abdulsalam Sufyaan Hamed Mustafa Jumaah Abbas Zahraa ShehabAbdulsalam SNo ratings yet
- Standard T Parameter Estimate Error Statistic P-Value: Analysis of VarianceDocument9 pagesStandard T Parameter Estimate Error Statistic P-Value: Analysis of VarianceSalah-Eddine SaidiNo ratings yet
- Kurva Kesetimbangan Campuran Etanol-Air Kurva Kesetimbangan Campuran Etanol-AirDocument43 pagesKurva Kesetimbangan Campuran Etanol-Air Kurva Kesetimbangan Campuran Etanol-AirBASNo ratings yet
- CHE317 Excel Lect4Document32 pagesCHE317 Excel Lect4Ub UsoroNo ratings yet
- Half-Wave RL Circuit With A Free-Wheeling DiodeDocument7 pagesHalf-Wave RL Circuit With A Free-Wheeling Diodehamza abdo mohamoud100% (2)
- Department of ChemistryDocument15 pagesDepartment of ChemistryWorcPrimerNo ratings yet
- 25 Petrucci10e CSMDocument25 pages25 Petrucci10e CSMAlexNo ratings yet
- KinetikaDocument8 pagesKinetikaDian Puspita SariNo ratings yet
- Part 3: RK Equation:: - 1 RT/P (1+B/ - 0+C/ - 2)Document4 pagesPart 3: RK Equation:: - 1 RT/P (1+B/ - 0+C/ - 2)nnbNo ratings yet
- Virial ExpansionsDocument4 pagesVirial ExpansionsnnbNo ratings yet
- Virial ExpansionsDocument4 pagesVirial ExpansionsnnbNo ratings yet
- Assigment1 SolDocument5 pagesAssigment1 SolMahmood SamadpoorNo ratings yet
- Gaussian Input for PCMDocument26 pagesGaussian Input for PCMManoel MachadoNo ratings yet
- Vapor/Liquid Equilibrium: Vle by Modified Raoult'S LawDocument16 pagesVapor/Liquid Equilibrium: Vle by Modified Raoult'S LawAby JatNo ratings yet
- 4 ProblemsDocument17 pages4 Problemssarath83277% (13)
- RC AND RL CIRCUIT RESPONSEDocument33 pagesRC AND RL CIRCUIT RESPONSESyed Muhammad DanishNo ratings yet
- Distillation Column - VLE Estimation and Operating LinesDocument5 pagesDistillation Column - VLE Estimation and Operating LinesKvspavan KumarNo ratings yet
- Centrifugal CompressorDocument7 pagesCentrifugal CompressorArturo VMNo ratings yet
- Fundamental of Power Electronics Week 8 Assignment SolutionsDocument4 pagesFundamental of Power Electronics Week 8 Assignment SolutionsDeep Gandhi100% (1)
- RELIEF VALVE PARAMETERSDocument6 pagesRELIEF VALVE PARAMETERSMa AlNo ratings yet
- Numerical Analysis of Engineering SystemsDocument16 pagesNumerical Analysis of Engineering SystemsCKNo ratings yet
- Rahul Chakraborty (191-036-801) EME Lab Report Document (1527) PDFDocument7 pagesRahul Chakraborty (191-036-801) EME Lab Report Document (1527) PDFNeFariOus ArYan AraFat AnikNo ratings yet
- Step 1 To Collect DataDocument4 pagesStep 1 To Collect DataZain Ul AbedinNo ratings yet
- Distillation Tower DesignDocument66 pagesDistillation Tower DesignHavocFireNo ratings yet
- Equarion of State - ExcelDocument4 pagesEquarion of State - Excelchemicaly12No ratings yet
- (Type The Document Title) : Thermodinamika Teknik KimiaDocument4 pages(Type The Document Title) : Thermodinamika Teknik KimiaonyuNo ratings yet
- Calculated DEQ Variables for Exothermic ReactionDocument7 pagesCalculated DEQ Variables for Exothermic ReactionHong KaewtipNo ratings yet
- Adobe Scan 02 Nov 2023Document15 pagesAdobe Scan 02 Nov 2023soniakhatuna1985No ratings yet
- Assignment FINALDocument67 pagesAssignment FINALlaila khanNo ratings yet
- Villanueva, Loise Bryan P. (III-EA)Document4 pagesVillanueva, Loise Bryan P. (III-EA)Jessica BalateroNo ratings yet
- Examples ExamplesDocument14 pagesExamples ExamplesWang SolNo ratings yet
- Distillation Tower DesignDocument65 pagesDistillation Tower DesignAntonio SilvaNo ratings yet
- Solution Manual Elements of Chemical Reaction Engineering 4th Edition WWW - Elsolucionario.org 573 680Document108 pagesSolution Manual Elements of Chemical Reaction Engineering 4th Edition WWW - Elsolucionario.org 573 680Jose Maria Quintas GironNo ratings yet
- Periodic and Impulsive Loading ResponseDocument10 pagesPeriodic and Impulsive Loading ResponseChí Khang ĐặngNo ratings yet
- Bottom Hole PressureDocument5 pagesBottom Hole PressureCHANADASNo ratings yet
- CHE3161 - Semester1 - 2010 - SolutionsDocument14 pagesCHE3161 - Semester1 - 2010 - SolutionsvenkieeNo ratings yet
- DistillationDocument23 pagesDistillationGueule D'angeNo ratings yet
- From Table 3.1: Parameter Assignments For Vdw Eos: Of "Introduction To Chemical Engineering Thermodynamics" (Smith And Van Ness) Α (T) ΣDocument4 pagesFrom Table 3.1: Parameter Assignments For Vdw Eos: Of "Introduction To Chemical Engineering Thermodynamics" (Smith And Van Ness) Α (T) Σjimiannah25No ratings yet
- Watercolor Painting Art Ebooks - Beginning - Landscape-Portrait - Animals - Urban - Trees-Flowers and ....Document1 pageWatercolor Painting Art Ebooks - Beginning - Landscape-Portrait - Animals - Urban - Trees-Flowers and ....jhon smithNo ratings yet
- Prob. 3.101 DataDocument1 pageProb. 3.101 DatajarotNo ratings yet
- Prob. 2.102 DataDocument1 pageProb. 2.102 DatajarotNo ratings yet
- Prob. 3.104 DataDocument1 pageProb. 3.104 Datajhon smithNo ratings yet
- Prob. 5.126 DataDocument1 pageProb. 5.126 DatajarotNo ratings yet
- Prob. 2.105 DataDocument1 pageProb. 2.105 Datajhon smithNo ratings yet
- Prob. 2.104 DataDocument1 pageProb. 2.104 Datajhon smithNo ratings yet
- Cate NaryDocument2 pagesCate Naryjhon smithNo ratings yet
- Prob. 10.100 DataDocument1 pageProb. 10.100 Datajhon smithNo ratings yet
- Srinagr Power ChanelDocument3 pagesSrinagr Power Chaneljhon smithNo ratings yet
- Section PropertiesDocument1 pageSection Propertiesjhon smithNo ratings yet
- Prob. 10.99 DataDocument1 pageProb. 10.99 Datajhon smithNo ratings yet
- Prob. 1.91 DataDocument1 pageProb. 1.91 Datajhon smithNo ratings yet
- Cate NaryDocument2 pagesCate Naryjhon smithNo ratings yet
- Equivalent Length MethodDocument1 pageEquivalent Length MethodbryesanggalangNo ratings yet
- Critical Flow Sub-Critical FlowDocument4 pagesCritical Flow Sub-Critical Flowjhon smithNo ratings yet
- Naps Gas RateDocument2 pagesNaps Gas Ratejhon smithNo ratings yet
- HP Energy CalcDocument1 pageHP Energy Calcjhon smithNo ratings yet
- pc28 003Document47 pagespc28 003jhon smithNo ratings yet
- Piping Loss Calculations: Input Values Head Loss, Given Piping and FlowDocument1 pagePiping Loss Calculations: Input Values Head Loss, Given Piping and Flowjhon smithNo ratings yet
- E-Books & PapersDocument1 pageE-Books & Papersjhon smithNo ratings yet
- pc28 001Document6 pagespc28 001jhon smithNo ratings yet
- Ma25 001Document18 pagesMa25 001jhon smithNo ratings yet
- 3020 PDFDocument1 page3020 PDFjhon smithNo ratings yet
- 3022 PDFDocument1 page3022 PDFjhon smithNo ratings yet
- Dolphin Gas Field Development CONTRACT 303740/01 - L2368Document1 pageDolphin Gas Field Development CONTRACT 303740/01 - L2368jhon smithNo ratings yet
- pc28 002Document10 pagespc28 002jhon smithNo ratings yet
- 3017Document19 pages3017jhon smithNo ratings yet
- BKDD00-ME-1V-00-016 Rev0 Technical BID Evaluation For Flare PackageDocument56 pagesBKDD00-ME-1V-00-016 Rev0 Technical BID Evaluation For Flare Packagejhon smithNo ratings yet