Professional Documents
Culture Documents
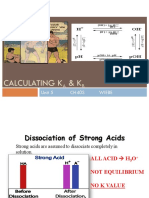
Acid-Base Strength: H O +H O H O+H O
Uploaded by
Melvin Edixon Salazar VásquezOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Acid-Base Strength: H O +H O H O+H O
Uploaded by
Melvin Edixon Salazar VásquezCopyright:
Available Formats
Acid-Base Strength
→ - + [H+][CN-]
HCN +H2O ← CN + H3O Ka = [HCN]
HCN → +
← H + CN
-
- → - [HCN][OH-]
CN + H2O ← HCN + OH Kb = [CN-]
→ + - [H+][A-]
HA ← H +A Ka = [HA]
[HA][OH-]
A- + H2O →
← HA + OH
-
Kb =
[A-]
[H+][A-] [HA][OH-]
Ka Kb = [HA] = [H+][OH-] = Kw
[A-]
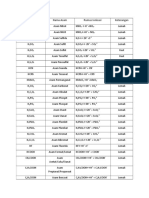
acid Ka Conjugate Kb
base
HClO4 HClO4+H2O → ClO4-+H3O+ ClO4-
H2SO4 HSO4-
HCl ~105 Cl-
HNO3 ~24 NO3-
H3O+ 1 (H3O++H2O → +
← H2O+H3O ) H2O 1x10-14
HSO4- 1.0x10-2 SO42- 1.0x10-12
H3PO4 7.1x10-3 H2PO4- 1.4x10-12
HOAc 1.8x10-5 OAc- 5.6x10-10
HCN 6.2x10-10 CN- 1.6x10-5
NH4+ 5.7x10-10 NH3 1.8x10-5
HPO42- 7.1x10-13 PO43- 0.014
H2O 1x10-14 OH- 1 (OH-+H2O → -
← H2O+OH )
NH3 NH2- NH2-+H2O → ← NH3+OH
-
OH- O2- O2-+H2O → -
← OH +OH
-
You might also like
- Exploration of Low Sulfidation Pithermal Vein Systems: By: Dr. Peter MegawDocument5 pagesExploration of Low Sulfidation Pithermal Vein Systems: By: Dr. Peter MegawMelvin Edixon Salazar VásquezNo ratings yet
- QGIS MinExpln Draft 202009Document169 pagesQGIS MinExpln Draft 202009Melvin Edixon Salazar Vásquez100% (1)
- Acid-Base TitrationDocument150 pagesAcid-Base TitrationKukkiboNo ratings yet
- LEAPFROG 4.1 Manual - Basico-Intermedio - EnglishDocument133 pagesLEAPFROG 4.1 Manual - Basico-Intermedio - EnglishMelvin Edixon Salazar VásquezNo ratings yet
- 1-Neutralization Theory2Document24 pages1-Neutralization Theory2watersoul.nNo ratings yet
- Basic Chemistry - Final NotesDocument1 pageBasic Chemistry - Final NotesJerry G0% (1)
- Tarea 5 Ácidos y Bases MonopróticosDocument2 pagesTarea 5 Ácidos y Bases MonopróticosSantiago GarciaNo ratings yet
- Tarea 5 Ácidos y Bases MonopróticosDocument2 pagesTarea 5 Ácidos y Bases MonopróticosEilene Valentina Perez NeraNo ratings yet
- Skoog - Solucionário Capítulo 9Document27 pagesSkoog - Solucionário Capítulo 9Thais Dos Santos100% (1)
- BOOKLET - Chemsheets A2 009 (Acids - Bases)Document21 pagesBOOKLET - Chemsheets A2 009 (Acids - Bases)sophieNo ratings yet
- Screenshot 2023-10-08 at 5.58.10 PMDocument6 pagesScreenshot 2023-10-08 at 5.58.10 PMAhmad Abo QadouraNo ratings yet
- Tobias, Mark Denzel B. Bse 1B Science: Worksheet - Bronsted-Lowry Acids and Bases Name: Year and Section: DateDocument2 pagesTobias, Mark Denzel B. Bse 1B Science: Worksheet - Bronsted-Lowry Acids and Bases Name: Year and Section: DateMaden betoNo ratings yet
- 09Document12 pages09ZenPhiNo ratings yet
- A Redox Reactions or Not) ANS Smyu53Document2 pagesA Redox Reactions or Not) ANS Smyu53ams13slaysNo ratings yet
- Hóa Phân Tích - Chap 2. Acid-Base EquilibriumtitrationDocument88 pagesHóa Phân Tích - Chap 2. Acid-Base Equilibriumtitrationnguyenthibaongoc20051No ratings yet
- Chap 2. Acid-Base Equilibrium&titrationDocument86 pagesChap 2. Acid-Base Equilibrium&titrationNgọc Việt NguyễnNo ratings yet
- Equilibrio Ácido-Base - Sistemas Ácido-Base MonopróticosDocument13 pagesEquilibrio Ácido-Base - Sistemas Ácido-Base Monopróticosgabriela tapiasNo ratings yet
- Ebook Chemistry For Today General Organic and Biochemistry Hybrid Edition 8Th Edition Seager Solutions Manual Full Chapter PDFDocument55 pagesEbook Chemistry For Today General Organic and Biochemistry Hybrid Edition 8Th Edition Seager Solutions Manual Full Chapter PDFhaogwyneth050p96100% (11)
- Chemistry For Today General Organic and Biochemistry Hybrid Edition 8th Edition Seager Solutions ManualDocument34 pagesChemistry For Today General Organic and Biochemistry Hybrid Edition 8th Edition Seager Solutions Manualwhateverluminarycx9100% (27)
- ch17 PDFDocument61 pagesch17 PDFHafidz RafiqiNo ratings yet
- Calculating K & K: Unit 5 CH40S WiebeDocument15 pagesCalculating K & K: Unit 5 CH40S Wiebemichmart19No ratings yet
- (Benjamin, Chapt. 3) : Acids & BasesDocument13 pages(Benjamin, Chapt. 3) : Acids & BaseswastequestNo ratings yet
- Iodine ClockDocument42 pagesIodine ClockNino Jay FabrosNo ratings yet
- Rumus Asam Nama Asam Rumus Ionisasi KeteranganDocument4 pagesRumus Asam Nama Asam Rumus Ionisasi Keteranganelsa noviyantiNo ratings yet
- Selective Reductions by Different Reducing AgentsDocument1 pageSelective Reductions by Different Reducing Agentsshankaranand200517No ratings yet
- 3 Equilibrio Ácido-Base (1) - Sistemas Ácido-Base MonopróticosDocument13 pages3 Equilibrio Ácido-Base (1) - Sistemas Ácido-Base MonopróticosDaniela LandinezNo ratings yet
- 02 Neutralization Reactions Problem Set 2Document2 pages02 Neutralization Reactions Problem Set 2Jonghyun (Justin) YangNo ratings yet
- (Benjamin, Chapt. 3) : Acids & BasesDocument13 pages(Benjamin, Chapt. 3) : Acids & BasesNermeen ElmelegaeNo ratings yet
- 4-Acid-Base 1Document32 pages4-Acid-Base 1José de Jesús Treviño ReséndezNo ratings yet
- Chapter 3 - Concept of Acid-Base NeutralisationDocument58 pagesChapter 3 - Concept of Acid-Base NeutralisationIkmal FikriNo ratings yet
- Chapter 14 - Acids - bases-pH-pOH and Buffers-AggiesDocument72 pagesChapter 14 - Acids - bases-pH-pOH and Buffers-AggiesShakira AntiquinaNo ratings yet
- W2 - Chemical Base Life - Chapter2Document17 pagesW2 - Chemical Base Life - Chapter2Ahmed AlbaderNo ratings yet
- Lecture 14 Acids and BasesDocument79 pagesLecture 14 Acids and BasesDuy Do MinhNo ratings yet
- Tarea 4 Tratamiento Sistemático Del Equilibrio QuímicoDocument1 pageTarea 4 Tratamiento Sistemático Del Equilibrio QuímicoSantiago GarciaNo ratings yet
- Tarea 4 Tratamiento Sistemático Del Equilibrio QuímicoDocument1 pageTarea 4 Tratamiento Sistemático Del Equilibrio QuímicoEilene Valentina Perez NeraNo ratings yet
- 02 - Kiseline, Baze, SoliDocument27 pages02 - Kiseline, Baze, SolispicybicNo ratings yet
- Chemistry Practice Exercise by Lemuel QueenDocument26 pagesChemistry Practice Exercise by Lemuel QueenPOPPY OFFICIALNo ratings yet
- APChemDocument8 pagesAPChemMacie CareyNo ratings yet
- NH 3 H 2 o Oh NH 4 Acid Base Concepts Chapter 15 H Conjugate Acid Base Pairs H Base AcidDocument12 pagesNH 3 H 2 o Oh NH 4 Acid Base Concepts Chapter 15 H Conjugate Acid Base Pairs H Base AcidKhang TrầnNo ratings yet
- Nathaniel Herod - BalancingpracticeDocument10 pagesNathaniel Herod - BalancingpracticeNathaniel HerodNo ratings yet
- Kesetimbangan Asam Basa Dan BufferDocument30 pagesKesetimbangan Asam Basa Dan BufferNashiruddin AlifNo ratings yet
- Balancing Equations: Practice Problems: Equation Balancing Chemistry Assignment: No 1Document4 pagesBalancing Equations: Practice Problems: Equation Balancing Chemistry Assignment: No 1Sher KhanNo ratings yet
- 2020-I-Marcha Sistematica I-Ii-IiiDocument2 pages2020-I-Marcha Sistematica I-Ii-IiiAdrianQuispeNo ratings yet
- Ebook Chemistry 10Th Edition Whitten Solutions Manual Full Chapter PDFDocument37 pagesEbook Chemistry 10Th Edition Whitten Solutions Manual Full Chapter PDFJaniceMarqueznxed100% (13)
- Balancing Questions PracticeDocument5 pagesBalancing Questions PracticeZunaira SafdarNo ratings yet
- Balancing Questions PracticeDocument5 pagesBalancing Questions PracticeZunaira SafdarNo ratings yet
- Asam Dan Basa Serta Kesetimbangan Asam Dan BasaDocument74 pagesAsam Dan Basa Serta Kesetimbangan Asam Dan BasaDinda FirdasariNo ratings yet
- Topic 12 NotesDocument31 pagesTopic 12 NotesHassan 2No ratings yet
- INzicht - Examencommissie - Cursus - Biologie 3ASODocument220 pagesINzicht - Examencommissie - Cursus - Biologie 3ASOMohamed Nemmouh OmarNo ratings yet
- Ch18Acid Base (A)Document45 pagesCh18Acid Base (A)Michael Conan MaglaqueNo ratings yet
- Diagnostics of Argon Injected Hydrogen Peroxide Added High Frequency Underwater Capillary DischargeDocument13 pagesDiagnostics of Argon Injected Hydrogen Peroxide Added High Frequency Underwater Capillary DischargeLeonardo MonteiroNo ratings yet
- Balancing Equations Chemical EquationsDocument10 pagesBalancing Equations Chemical EquationsBRIAN NYASULUNo ratings yet
- C Pourbaix, Dist, Bjerrum, P and MDocument8 pagesC Pourbaix, Dist, Bjerrum, P and MWinter HunterNo ratings yet
- Aqueous Chemistry Lecture 2 & 3Document81 pagesAqueous Chemistry Lecture 2 & 3NEELAMNo ratings yet
- Tabele Chimie Analitica Grupele 1 2 Si 4Document38 pagesTabele Chimie Analitica Grupele 1 2 Si 4I IiNo ratings yet
- PRESENTASI - Salt Hydrolysis and ExercisesDocument34 pagesPRESENTASI - Salt Hydrolysis and ExercisesSalim Sanjaya100% (1)
- Ebook Chemical Principles The Quest For Insight 7Th Edition Atkins Solutions Manual Full Chapter PDFDocument67 pagesEbook Chemical Principles The Quest For Insight 7Th Edition Atkins Solutions Manual Full Chapter PDFJaniceMarqueznxed100% (11)
- 1 - Introduction To Acid-Base PDFDocument4 pages1 - Introduction To Acid-Base PDFJron Victor Smith SamsonNo ratings yet
- CHE Rovnovazna A Disociacna KonstDocument1 pageCHE Rovnovazna A Disociacna KonstDaniela SuránováNo ratings yet
- Topic 12 NotesDocument31 pagesTopic 12 NotesHK Nova ChiuNo ratings yet
- Balancing EquationsDocument6 pagesBalancing Equationssyed abdul ahadNo ratings yet
- Constantes de Ionizacion de Acidos Debiles KaDocument3 pagesConstantes de Ionizacion de Acidos Debiles KaLaime Tancara Miguel ÁngelNo ratings yet
- UntitledDocument5 pagesUntitledMelvin Edixon Salazar VásquezNo ratings yet
- William D. Nesse - Introduction To Mineralogy-Oxford University Press (1999)Document457 pagesWilliam D. Nesse - Introduction To Mineralogy-Oxford University Press (1999)Melvin Edixon Salazar VásquezNo ratings yet
- Are You Listening Prefers Knows Is Making Need Is Studying Am Working Use Are - Wearing Are - LikingDocument2 pagesAre You Listening Prefers Knows Is Making Need Is Studying Am Working Use Are - Wearing Are - LikingMelvin Edixon Salazar VásquezNo ratings yet
- Vectores Espacio 8Document1 pageVectores Espacio 8Melvin Edixon Salazar VásquezNo ratings yet
- Windows 8.1 Phone Activation Mak-Retail KeysDocument1 pageWindows 8.1 Phone Activation Mak-Retail KeysMelvin Edixon Salazar VásquezNo ratings yet
- Windows 8.1 Phone Activation Mak-Retail KeysDocument1 pageWindows 8.1 Phone Activation Mak-Retail KeysMelvin Edixon Salazar VásquezNo ratings yet