Professional Documents
Culture Documents
THE BY: D U L Y-Corrosion Steel
Uploaded by
Steve OoiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
THE BY: D U L Y-Corrosion Steel
Uploaded by
Steve OoiCopyright:
Available Formats
304 DUL Y-CORROSION OF STEEL J.S.C.I.
, 6g, October, 1950
Acknowledgments After filtration through a Berkfeld candle, sea water was
Acknowledgments are due to Prof. Sir Ian Heilbron; with evaporated until crystals separated and the evaporation was con-
whose cooperation the work was carried out a t The Imperial tinued on a water bath until a fairly dry mass of crystals was
College of Science and Technology; to Mr. J. E. Youell, obtained, the temperature at no time exceeding 80"c. T h e
who assembled the results and prepared the paper for publica- crystals were then dried for 36 hr. in a vacuum desiccator over
tion; and to the Directors of The Distillers Company Ltd. concentrated sulphuric acid at room temperature, then powdered
for permission to publish the results. in a mortar and dried in a vacuum for a further 6 hr. 20 g.
of the sample was weighed out iuto a glass tube and sealed
T h e Distillers Company Ltd. at once.
Research and Development Department The same technique as above33 .I was used to determine the
Great Burgh, Epsom percentage of moisture absorbed by the dried sea salt after it
Surrey had reached equilibrium with air of known relative humidity.
Ikccired Xorcmher 7, 19.19 Samples of the salt (about 2 g.) were weighed out on crucible
lids suitably wired up to be suspended from the beam of a
References Beeker Chainomatic balance. T h e samples were then suspended
1 Reboul, Aiai. Chim 1878,(s), 14, 462 over sulphuric acid of appropriate densities to maintain atmo-
2 Heincmnnn, U.S.P. 1,180,947 ; C h i . Abs. 1916, 10, 1693
Du Pont d e Ncmours, U.S.P.1,477,047
spheres of 10, 20, 30, 35,40, 50,60,70 and 80 per cent relative
I Klebanski and Wolkenstcin, Chew. Zeiitr. 1935, 11. 3298
humidity in Kilner jars, the jars being kept in a room maintained
Carbide and Carbon Chem. Corpn., U.S.P. 1,75s,o4g constantly at 24O c. (75' F.). At intervals the samples were
(I Shell Development Co., U.S.P. z,207,1g3; Clieiw. Abs. 1940,34. reweighed in the constant temperature room without removing
19:4
Mugdan, M.and Barton, D. H. R., B.P. 573,532
them from the jars. The experimental results are shown in
h Goodcrham, W. J., J. SOC.c H ~ h i IND.
. I935,54,297T Table I and graphically in Pigs. I and 2. T h e sample exposed
to air at 307" R.H. lost weight in reaching equilibrium, whereas
that at to:{, R.H.lost slightly more. These losscs probably
correspond to the amount of moisture taken up by the sample
during its initial transfer from the sealed tube at the time of
THE CORROSION OF STEEL BY SEA the first weighing, but samples kept a t 357" R.H.and above
SALT OF GIVEN MOIS'PURE CONTENT increased in weight rapidly during the first 24 hr.
When the samples of sea salt prepared in this way are brought
Dg S . J. DULY into contact with bright steel plate and kept a t the relative
T h e moisture content of the residue obtained by evaporating sea humidity appropriate to the condition of the salt, the tempera-
water is shown herc to be determined by the rclativc humidity of ture being kept constant, the steel is corroded away fast by the
the air In which it is subscquenrl kept, provided that the storage damp salt but remains almost (and perhaps entirely) unaffccted
temperature does not vary : sampLs of such sea salt corrode mild
steel in proportion to their moisture contcnts. It follows that the by the salt that is quite dry.
w e n t ofcorrosion of mild steel by sea salt is governed by the relative
humidity of the ambient air and that keeping the moisture content
of the salt-complcx 81 S",, or less subsrantially prevents corrosion
from this cause.
s
One of the urgent problems confronting those responsible $45- -
for tanker design and operation is the prevention of corrosion d
of the interiors of the tanks.' The corrosion is due in part
to the action of sea water used as ballast or for the purpose of
cleaning out the tanks, and in part to the nature of the cargo.
The present paper is concerned with that part of thc corrosion
due to the sea water. Air dried artificially by large silica-gel
driers has already been used to dry out empty tanks after they
have been cleaned with sea water, or emptied of sea-water
ballast.' The effectiveness of this procedure depends, among
other things, on the amount of moisture remaining in the dried-
out sea saIt when conditions of equilibrium are reached.
!
X 2+/
a
W
Whereas the moisture content of many natural products
kept at constant temperature has been determined in its relation
to the humidity of the air to which the product is exposed, sea
salt has not hitherto been the subject of experiment. T h e tech-
nique for this purpose is, however, well-established and has
been described by L. H. N. Cooper and H. Daynes' and used
by various investigators? This determination was thc first
step in the present investigation.
Sea salt for thc purpose was prepared by the Plymouth Lab-
oratory of the Marine Biological Association, using sea water
from the English Channel. Bearing in mind the complex nature
of the residue when sea water is evaporated,5 the following
procedure nas adopted to secure a product likely to react to
moisture in the same way as the residue which was. left when
sea water dried out on the surface. Thc method adopted by
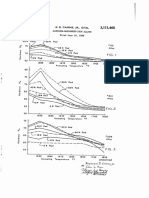
the Association for the preparation of sea salt was as FIG. I. Absorptioii of iiioisriire by oacino,r-dried sea salt at a coiIsIaiiI
follows : rettiperarcire of 75" P.
J.S.C.I., @, October, 1950 DUL Y-CORROSION OF STEEL 305
the corresponding constant relative humidities in a constant
, temperature room. At the end of 13 days, the salt was trans-
*' ferred from the driest samples into standard 50 ml.-flasks
without washing. A glass rod was used to assist in transferring
the damper samples, and the cups washed out with distilled
water intoa IOO m1.-flask.
Immediately after transferring the contents of the cups to the
flasks, a solution of 15% by volume sulphuric acid was run in to
dissolve the corrosion products. The acid contained 0.05% di-
o-tolylthiourea to inhibit any attack on the steel itself. The
samples were then placed in an oven at 40" c. After 30 min. the
acid was run off into the appropriate flask, and the cup washed
with distilled water. The solutions thus obtained were analysed
for iron colorimetrically, with the results given in Table 11. The
results are also expressed graphically in Fig. 3. A comparison
Table I1
Wtiglir of irou corrodedJrum bright steel plate iii 13 days by sea salr con-
taitiiiig varyiiig perceiifager of vioistiire, wheir kept iii air at the relative
hiiniidities required to itiaiiiraiii eqiiilibriiiiii at 75" F.
Temperature 75" F.
R.H. of Moisture Iron removed Iron Iron
air, content of sea after 13 days removed, removed,
perccntagc salt (basis, dry contact with sea g./m' oz./ft.'
wt.), pcrccntage salt, mg./S*lr cm.'
80 231' 0 7'00 12'05 0'0394
70 38.4 1.55 4'39 0.0143
60 18.5 1'15 1'99 0.0063
50 8.1 0.92 1.58 0 . 0 0 5 2
45 5'9 0.56 0.96 0.0032
35 3 '45 0.48 0.82 0.0027
I 30 0'2 0'35 0.60 0'0020
MOISTURE
' d
ON DRY
0 2 3 h 5 ' 49 e'l
WEIGHT OF SEA
le'5 3d4
SALT, oer cent
2ilO 20 0 '0 0'17 0.47 0.0016
PIG.2. l'drceitragc iiicrease in rueiglir uf vaciiioli-dried sea salt exposed to air
of aarioiis relative Iiiotiidiiies wider static coiidirioiis at a coiistaiit rerupera-
trire of 75" P.
Table I
Change iit weigh of vacriiiiii-dried sra-salt wlteir exposed to air at variorrs
relative Aiieiidirier at cuiistuuf rctnpernture ticider static coitditioiir
Tempcraturc 24' C. (75" P.)
K.H. of Percentage increase or Appearance of salt
illr, dccrcnsc after after 27 days
Pcrccntogr I day 8 days 27 days
80 +40.70 +155'0 j 1 3 2 . 0 Clear liquid; one particle
of undissolved salt rc-
mained
70 i-19.81 + 37.4 -t 39'2 Mainly damp solid, but
with free liquid
+
60
50 +i 1 37. .7 58 ~ -i--I- 19'3
8.9 +
J9.3 Slushy
8 . 9 Damp and cakcd
1; $ ;:;g $ 4:: ;;:; ;;;fK-::y:,$ilere [ O h
glass rod
35 + 2.99 -1- 4.25 + 4'25 Dryglassbutrodwill adhere to a
30 - 0.08 - 0.1 - 0.1 dry andfree-running
20 - 0.50 - 0.69 - 0.8 Dry and frcc-running
The steel samples for the above purpose measured approsi-
matcly 2.8 cm. square by 0.3 cm. thick. They were polished
bright on all sides with fine emery, cleaned and lacquercd.
Thc lacquer was then reniovcd from one side with emery and
a glass ring I cm. deep and 2 . 72 cm. internal diameter cemented
to the steel with a plastic cement. The bright steel outside the
ring was also cemented, so that only that within the ring was
free to rust. The samples of salt from the previous experiment
were transferred to the cups thus formed so that they were FIG,3. IVekIir of iroii reinoved froui brkh steel plate bi 13 days at 75" F.
in contact with the steel. They were then suspended in air at rvkeri kept iii coiitact with sea salt coiiruiriitig krroruir anioiim of ittoistiire
306 THOMAS A N D IVILKINSON-CARBON DIOXIDE IN C O A L J.S.C.I., 6g, October, 1950
with the curve in Fig. 2 shows that the amount of corrosion obtained by reaction of the coal with dilute hydrochloric acid
depends upon the moisture content of the salt which in turn or phosphoric acid is drawn into a 24-1. evacuated flask where
depends on the relative humidity of the air under the conditions barium hydroxide is added, and the excess barium hydroxide
of the experiment. The amount of corrosion taking place at is titrated with hydrochloric acid. This method specifies
60O,:,R.H. is only one sixth that at 80% R.H. heating at 50" C. for 30 rnin., and a t 100' C. for 10 min. ; the
The samples of steel were examined under a binocular reaction with barium hydroxide takes 30 min.
magnifier when the experiment was finished. T h e sample In Fuel Research Survey Paper No. 44 a gravimctric mechod
which had been kept at 20'1: R.H. showed no sign of corrosion. is described in which the carbon dioxide liberated is absorbed
T h e small quantity of iron found in the acid solution was in indicator-type soda-lime and determined gravimetrically.
probably dissolved as iron in spite of the inhibitor. T h e weight Heating is for 15,min. to the boiling point, with gentle boiling
of iron shown as removed at zo'z, R.H. in Fig. 3 and Table I1 for 3-35 min.
would then be nil and the values of other percentages relative Hughes' has described a method for determining carbon
humidity would require to be adjusted accordingly. T h e sample dioxide in baking powder, in which the pressure of the carbon
exposed to air at 30"/~ R.H. showed no sign of corrosion either. dioxide, enclosed in an apparatus of constant volume, was
But that exposed to air at 3 5 7 , R.H. showed slight etching in measured and the percentage of carbon dioxide read from a
specks, visible also to the naked eye. That at 45y;, R.H. calibration curve after 3 min. With the materials that were
showed similar etching but more general. The sample at 50°<, investigated this method gave rapid estimation in the cold.
R.H. showed deeper sporadic etching. That at 60%~ R:H. The solubility of carbon dioxide in the acid is minimized by
had lost iron from the whole surface; so had the remaining using low pressures in the apparatus. This method was applied
two samples at 70",;, and So":, R.H. to dusts from mine roads by the Monmouthshire and South
The curve (Fig. 3) corresponds to the case examined by \Vales Coal Owners' Association? and was found to take about
W. H. J. Vernon'; in which rapid corrosion of iron was shown 54 min. per sample.
to set in at a temperature above the dew-point through thc Bee? applied Hughes' method to fluorspar, which was used
presence of liquid water owing to the hygroscopic nature of as a flux in open-hearth furnaces, as it was required to find out
the initiating solid and the resulting corrosion product. the quantity of calcite present. The method is extensively
The conclusion that may be drawn from the foregoing investi- used in the Sheffield steel works for this purpose. Beet also
gations is that corrosion of the inner surface of a tank by sea applied it to coals and found good agreement with values
salts would be materially slowed down by maintaining an artifi- obtained by the B.S. method for three coals. He reduced the
cially dry atmosphere within it. Although the curve in Fig. 3 volume of the flask containing the coal by using a 50-ml.
is smooth, so that strictly there is no point which could be suction flask (nominal volume to the side arm) ; he used glass
described as corresponding to a ' critical ' R.H. value, the rate of beads to standardize the volume of a series of flasks to use with
corrosion under the conditions of the experiment increases the same U-gauge ; he put indicating-type silica gel as a desic-
rapidly as the moisture content of the salt rises above St%,. cant in a 4-mm. bore tube instead of the wider tube used by
T h e practical aim would, therefore, appear to be to dry out the other workers, and added a silica gel guard-tube before the
surface until these conditions are rcachcd. two-way tap connected to the water pump and the atmosphere.
He also increased the bore of the upper part of thc capillary
Cargocaire Limited gauge from I to 4 mm., thus nearly doubling the reading.
I 55 Fenchurch Street He also advised heating of dolomite materials over a smalL
London, E.C.3 bunsen flame for 2-3 rnin., and cooling subsequently in a
Hrrci%cd.\kip 1 5 , 1950
beaker of water for 7 rnin., the whole determination taking
15-20 min.
References The effect of a number of different factors has been studied
' Lamb, J.,J. l i t s f . l'crrol. 1949,35, 339 using the apparatus illustrated in Fig. I. This is the same in
Colvin. 0. D., SOC.,taw. Archirccrs i w r . Ewrs.,
- . Philadelphia Chaptcr principle as that used by Hughes and the other workers men-
OCt. 1949 tioned, but is a true constant-volume apparatus in that it
3 Dames. H.. 7. Riibb. Res. 1936,5, 131
Gnhc, R., J:"Soc. CHEM. IND. 194r, 5 & ~ 4 omits the funnel with tap for adding the acid. From a funnel
j Phillios. H. C.,C/teiit. Soc. Qriarr. Rev. 1947, 1, 9 of this type it is difficult to obtain the same volume of acid in
Vern&; W. H.. J., 1'ram. FaradaJI. Sot. 1927,23, 162 ; 1931,27,2Ss all tests, or to avoid in-leakages of air if all the acid is added.
I n the apparatus described here the acid is spilt from an internal
container as in the well-known method for determining CO,
THE DETERMINATION OF CARRON in carbonates. Since it has been found that heat has to be
DIOXIDE IN COAL applied to break up the small amount of carbonates present in
coal, it is easier to manipulate the small reaction flask without
Ug W. C. THOMAS and )I. C. \I'ILYINSON a tap funnel. A convenient reaction flask is a 50-1111. CO,
In the present Br;tish Standard method for the determination of flask fitted with a rubber stopper which is always inserted to
carbon dioxide in coals, thc carbon dioxide evolved by rcaction of the same level, marked by a scratch. Some of the tests were
the coal with dilute hydrochloric acid or phosphoric acid is absorbed carried out using a Ioo-ml. specific-gravity bottle with a wide
in barium hydroxide, and the excess barium hydroxide is titrated neck and a ground joint ending in a capillary ;with this appara-
with hydrochloric acid. In Fuel Research Survey Paper No. 44,
a method is described in which the carbon dioxide liberated IS tus the joint had to be lubricated freshly for each test. I n all
absorbed in soddime and determined gravimetically. In this tests a !-in. diameter flat-ended sample tube reduced to about
paper a third method based on the prcssomctric principle is des- I in. in length was used to hold the acid.
cribed. T h e amount of a r b o n dioxide is determined by measuring Tire effect of the voltirtre o/ the apparatus.-It was pointed out
the pressure developed in an apparatus of constant volume, the
pressure and volume relationship having previously been determined by the research workers of the Monmouthshire and South
using a known amount of sodium carbonate. Wales Coal Owners' Association that, in an apparatus of gas
The present B.S. method for the determination of carbon space V , the volume of air present at the initial pressure of PI
dioxide in coals uses Sinnatt and Harrison's modification of will decrease to 2 VP
when the pressure has increased to P..
the Schrotter apparatus; in this method the carbon dioxide P,
You might also like
- G2A Glitch DONT LEAK 2Document7 pagesG2A Glitch DONT LEAK 2qDeficiencyNo ratings yet
- The Practical Salinity Scale 1978 and Its AntecedentsDocument6 pagesThe Practical Salinity Scale 1978 and Its AntecedentsVinícius MartinsNo ratings yet
- Handbook - European Choral AssociationDocument24 pagesHandbook - European Choral AssociationMonica SaenzNo ratings yet
- The Effect of Overconsolidation On The Behaviour of Clays During Shear-HenkelDocument12 pagesThe Effect of Overconsolidation On The Behaviour of Clays During Shear-HenkelAnonymous GnfGTwNo ratings yet
- Preliminary Voters ListDocument86 pagesPreliminary Voters Listمحمد منيب عبادNo ratings yet
- Loan Agreement: Acceleration ClauseDocument2 pagesLoan Agreement: Acceleration ClauseSomething SuspiciousNo ratings yet
- Astm G5Document12 pagesAstm G5Mayra Lizeth Mayorga LaguadoNo ratings yet
- The Effect of Temperature On The Desorption of GoldDocument15 pagesThe Effect of Temperature On The Desorption of Goldcuberbill1980No ratings yet
- Reaction of PC With Carbon DioxideDocument6 pagesReaction of PC With Carbon DioxideNam HuynhNo ratings yet
- A Study of The Hydrothermal Growth of Ruby: BUTCHER, B.Se.Document12 pagesA Study of The Hydrothermal Growth of Ruby: BUTCHER, B.Se.Pulbere NeagraNo ratings yet
- Study On A Permanent Wall Type Converter With Water CoolingDocument11 pagesStudy On A Permanent Wall Type Converter With Water CoolingdsfdsNo ratings yet
- The Solubility of Carbon Dioxide in Calcium-Chloride-Water Solutions at 75, 100, 120 C and High PressureDocument5 pagesThe Solubility of Carbon Dioxide in Calcium-Chloride-Water Solutions at 75, 100, 120 C and High Pressuremoji20067147No ratings yet
- SDocument10 pagesSViet Quoc DinhNo ratings yet
- Phthalic Acid SolubilityDocument3 pagesPhthalic Acid SolubilityRajeshNo ratings yet
- The Diffusion of Hydrogen and Helium ThroughDocument8 pagesThe Diffusion of Hydrogen and Helium ThroughElenaNo ratings yet
- Simple Ozonizer: LaboratoryDocument1 pageSimple Ozonizer: LaboratoryAnonymous FigYuONxuuNo ratings yet
- Chlorine The Heat Capacity Vapor Pressure Heats ofDocument5 pagesChlorine The Heat Capacity Vapor Pressure Heats ofHusain MochammadNo ratings yet
- Modelling Co Solubility in Pure Water and Nacl-Type Waters From 0 To 300 8C and From 1 To 300 Bar Application To The Utsira Formation at SleipnerDocument13 pagesModelling Co Solubility in Pure Water and Nacl-Type Waters From 0 To 300 8C and From 1 To 300 Bar Application To The Utsira Formation at SleipnerzibaNo ratings yet
- On Acid (On: EffectDocument23 pagesOn Acid (On: Effectprakush01975225403No ratings yet
- The Study of Calcium-Sulphate Scale Pre Vention at Higher Steam Pressures1Document11 pagesThe Study of Calcium-Sulphate Scale Pre Vention at Higher Steam Pressures1DilipNo ratings yet
- 1 s2.0 S0010938X13005039 MainDocument4 pages1 s2.0 S0010938X13005039 MainDhanashekar ManickamNo ratings yet
- Heat Transfer Characteristics of Ice Melting in Water and Salt SolutionsDocument5 pagesHeat Transfer Characteristics of Ice Melting in Water and Salt SolutionsAlberto RosarioNo ratings yet
- WRRCTR 49Document56 pagesWRRCTR 49jodiNo ratings yet
- Relative Humidity-Temperature Relationships of Some Saturated Salt Solutions in The Temperature Range 0 To 50 CDocument8 pagesRelative Humidity-Temperature Relationships of Some Saturated Salt Solutions in The Temperature Range 0 To 50 Chendry taputraNo ratings yet
- 1993 Delayed Ettringite Formation A Microstructural and Microanalytical StudyDocument8 pages1993 Delayed Ettringite Formation A Microstructural and Microanalytical StudyDigo MeloNo ratings yet
- An Experimental Study of Cassiterite Solubility in HCl-bearing Water Vapor PDFDocument12 pagesAn Experimental Study of Cassiterite Solubility in HCl-bearing Water Vapor PDFNurjati SetiawanNo ratings yet
- Hydration Portland Cement Compounds: H. Lerch DDocument11 pagesHydration Portland Cement Compounds: H. Lerch DAnonymous uKccPQTNo ratings yet
- Halite Clogging in A Deep Geothermal Well - Geochemical and IsotopicDocument13 pagesHalite Clogging in A Deep Geothermal Well - Geochemical and IsotopicIsrael Arias GonzálezNo ratings yet
- Drilling Fluids For GeothermalDocument22 pagesDrilling Fluids For GeothermalAnonymous T32l1RNo ratings yet
- Roebuck 1942Document12 pagesRoebuck 1942Imam Saja DechNo ratings yet
- Methods For Calculating EvaporationDocument10 pagesMethods For Calculating EvaporationenviroashNo ratings yet
- Interstitial Water Chemistry of Anoxic LBNG Island Sound Sediments. 1. Dissolved Gases1Document16 pagesInterstitial Water Chemistry of Anoxic LBNG Island Sound Sediments. 1. Dissolved Gases1mscribariaNo ratings yet
- How Dry Is Dry SoilDocument6 pagesHow Dry Is Dry SoilAleksandar SpasojevicNo ratings yet
- Article PDFDocument4 pagesArticle PDFAdriana Flores DepazNo ratings yet
- The Diffusion of Gases Through Fused QuartzDocument6 pagesThe Diffusion of Gases Through Fused QuartzElenaNo ratings yet
- 1955 The System Ba (NO3) 2-KNO3Document3 pages1955 The System Ba (NO3) 2-KNO3Adrian CaraballoNo ratings yet
- H. D. Holland 1173: Input Transported by by Directly GiveDocument11 pagesH. D. Holland 1173: Input Transported by by Directly GiveGeorgiana BălaşNo ratings yet
- Changes in Silica Chemistry and Hydrology Across The Rotorua Geothermal Field, New ZealandDocument14 pagesChanges in Silica Chemistry and Hydrology Across The Rotorua Geothermal Field, New Zealandistiqomah mayaNo ratings yet
- SPE-2559-PA Shale Control With Balanced Activity ObmDocument8 pagesSPE-2559-PA Shale Control With Balanced Activity ObmWilliam RocaNo ratings yet
- Factors Affecting The Distribution of Silicate in The North Atlantic Ocean and The Formation of North Atlantic Deep WaterDocument16 pagesFactors Affecting The Distribution of Silicate in The North Atlantic Ocean and The Formation of North Atlantic Deep WaterAina AzlanNo ratings yet
- Freeze DryingDocument12 pagesFreeze DryingMy Pham Thi DiemNo ratings yet
- Methods For Calculating Brine Evaporation Rates During Salt ProductionDocument11 pagesMethods For Calculating Brine Evaporation Rates During Salt ProductionMarcusNo ratings yet
- The Practical Aspects Handling High-Pressure Sour Gas: R. Mottley and C. PfisterDocument6 pagesThe Practical Aspects Handling High-Pressure Sour Gas: R. Mottley and C. PfisterAhmadFauziNo ratings yet
- Bromley 1970Document8 pagesBromley 1970Muhammad Fakhar KhanNo ratings yet
- Display Article For FreeDocument4 pagesDisplay Article For FreeinfinitopNo ratings yet
- The Poisoning of Nickel Hydrogenation Catalysts by Water Vapor' 'Document2 pagesThe Poisoning of Nickel Hydrogenation Catalysts by Water Vapor' 'victor japposanNo ratings yet
- Ultra-Rapid Freezing of Water Treatment ResidualsDocument8 pagesUltra-Rapid Freezing of Water Treatment ResidualsRobert Meers DiazNo ratings yet
- DurstDocument7 pagesDurstRahul KatreNo ratings yet
- 1963 Metterties, Chemistry of BoranesDocument8 pages1963 Metterties, Chemistry of Boranesm222000No ratings yet
- RSPL 1900 0004Document5 pagesRSPL 1900 0004MihiretuNo ratings yet
- Impact of Temperature Difference (Water-Solar Collector) On Solar-Still Global EfficiencyDocument8 pagesImpact of Temperature Difference (Water-Solar Collector) On Solar-Still Global Efficiencyjkl. lkjNo ratings yet
- Diffusion of Helium Through QuartzDocument5 pagesDiffusion of Helium Through QuartzElenaNo ratings yet
- The Oceans PDFDocument8 pagesThe Oceans PDFLouie VerginoNo ratings yet
- The Effects of Initial TemperatureDocument5 pagesThe Effects of Initial TemperatureErhan Sedat EnerNo ratings yet
- White1971 - Vapor Dominated Hydrotermal Systems Compared With Hot-Water SystemDocument23 pagesWhite1971 - Vapor Dominated Hydrotermal Systems Compared With Hot-Water SystemNino PumaNo ratings yet
- The Hydrothermal Alteration of Feldspars in Acid Solutions Between 300 Degrees and 400 Degrees CDocument12 pagesThe Hydrothermal Alteration of Feldspars in Acid Solutions Between 300 Degrees and 400 Degrees Ccristian camilo guisao palacioNo ratings yet
- Franke Method CalciumDocument7 pagesFranke Method Calciumpriscila_hdzvNo ratings yet
- Hind 1954Document12 pagesHind 1954jgNo ratings yet
- The Solubility in Water of Ba, CA and MG Salts of Sulfamic AcidDocument5 pagesThe Solubility in Water of Ba, CA and MG Salts of Sulfamic AcidGabriela LodelaNo ratings yet
- Effect of Temperature On The Moisture So PDFDocument7 pagesEffect of Temperature On The Moisture So PDFJosé Manuel RodríguezNo ratings yet
- Spe 1199 0036 JPT PDFDocument2 pagesSpe 1199 0036 JPT PDFRaul Dolo QuinonesNo ratings yet
- Kinetics The Catalyzed and Uncatalyzed Liquid-Phase Hydration of PropyleneDocument7 pagesKinetics The Catalyzed and Uncatalyzed Liquid-Phase Hydration of PropyleneAlejandro HernandezNo ratings yet
- Design, Construction, Performance and Heat Extraction Studies of A Full Scale Non-Convecting Solar PondDocument33 pagesDesign, Construction, Performance and Heat Extraction Studies of A Full Scale Non-Convecting Solar PondAnuragSh1994No ratings yet
- Alkali-Silica Reaction and Delayed Ettringite Formation in Concrete - A Literature Review (0-4085-1)Document12 pagesAlkali-Silica Reaction and Delayed Ettringite Formation in Concrete - A Literature Review (0-4085-1)rahul dasNo ratings yet
- Scientific American Supplement, No. 455, September 20, 1884From EverandScientific American Supplement, No. 455, September 20, 1884No ratings yet
- Materials Corrosion - 2024 - Ooi - A New Index To Estimate The Corrosion Resistance of Aluminium Containing SteelDocument12 pagesMaterials Corrosion - 2024 - Ooi - A New Index To Estimate The Corrosion Resistance of Aluminium Containing SteelSteve OoiNo ratings yet
- Characterization of Electromagnetic Rotor Material Properties and Their Impact On An Ultra-High Speed Spinning Ball MotorDocument4 pagesCharacterization of Electromagnetic Rotor Material Properties and Their Impact On An Ultra-High Speed Spinning Ball MotorSteve OoiNo ratings yet
- 2018 4942 Moesm1 EsmDocument14 pages2018 4942 Moesm1 EsmSteve OoiNo ratings yet
- Knight Et Al 2005 Fatigue Life Improvement of Threaded Connections by Cold RollingDocument11 pagesKnight Et Al 2005 Fatigue Life Improvement of Threaded Connections by Cold RollingSteve OoiNo ratings yet
- N Series Thin-Section Ball Bearings: Metric and Inch DesignsDocument13 pagesN Series Thin-Section Ball Bearings: Metric and Inch DesignsSteve OoiNo ratings yet
- XPS Study of Passive Films Formed Ion An Iron-Aluminium Intermetallic Compound in Acid SolutionDocument7 pagesXPS Study of Passive Films Formed Ion An Iron-Aluminium Intermetallic Compound in Acid SolutionSteve OoiNo ratings yet
- Headley 2004Document24 pagesHeadley 2004Steve OoiNo ratings yet
- Örnek Et Al-2018-Npj Materials DegradationDocument15 pagesÖrnek Et Al-2018-Npj Materials DegradationSteve OoiNo ratings yet
- Olsson 1995Document13 pagesOlsson 1995Steve OoiNo ratings yet
- A Review of The Stainless Steel SurfaceDocument8 pagesA Review of The Stainless Steel SurfaceSteve OoiNo ratings yet
- A Review of The Influence of Environmental Humidity and W A T e R On Friction, Lubrication and WearDocument19 pagesA Review of The Influence of Environmental Humidity and W A T e R On Friction, Lubrication and WearSteve OoiNo ratings yet
- Basile 1993Document7 pagesBasile 1993Steve OoiNo ratings yet
- Need El Man 2014Document32 pagesNeed El Man 2014Steve OoiNo ratings yet
- Role of Surface Chemistry On The Nature of Passive Oxide Film Growth On Fe-Cr (Low and High) Steels at High TemperaturesDocument8 pagesRole of Surface Chemistry On The Nature of Passive Oxide Film Growth On Fe-Cr (Low and High) Steels at High TemperaturesSteve OoiNo ratings yet
- Tajammal Imran, Bo Jacobson, Asad ShariffDocument10 pagesTajammal Imran, Bo Jacobson, Asad ShariffSteve OoiNo ratings yet
- Criteria For The Formation of Protective Al O Scales On Fe-Al and Fe-Cr-Al AlloysDocument25 pagesCriteria For The Formation of Protective Al O Scales On Fe-Al and Fe-Cr-Al AlloysSteve OoiNo ratings yet
- US3111405 MnAlCFe SteelDocument7 pagesUS3111405 MnAlCFe SteelSteve OoiNo ratings yet
- Investigation of The Electrochemical Corrosion Behavior and Passive Film For Fe-Mn, Fe-Mn-Al, and Fe-Mn-Al-Cr Alloys in Aqueous SolutionsDocument10 pagesInvestigation of The Electrochemical Corrosion Behavior and Passive Film For Fe-Mn, Fe-Mn-Al, and Fe-Mn-Al-Cr Alloys in Aqueous SolutionsSteve OoiNo ratings yet
- Olsson 1995Document13 pagesOlsson 1995Steve OoiNo ratings yet
- Criteria For The Formation of Protective Al O Scales On Fe-Al and Fe-Cr-Al AlloysDocument25 pagesCriteria For The Formation of Protective Al O Scales On Fe-Al and Fe-Cr-Al AlloysSteve OoiNo ratings yet
- Recalibrated Equations For Determining Effect of Oil Filtration On Rolling Bearing LifeDocument32 pagesRecalibrated Equations For Determining Effect of Oil Filtration On Rolling Bearing LifeSteve OoiNo ratings yet
- Effect of Sulphur and Naphthenic Acids On The Corrosion of 9%Cr-1%Mo SteelsDocument9 pagesEffect of Sulphur and Naphthenic Acids On The Corrosion of 9%Cr-1%Mo SteelsSteve OoiNo ratings yet
- JP2004162119A - Corrosion Resistant Steel With Excellent Haz Toughness - Google PatentsDocument6 pagesJP2004162119A - Corrosion Resistant Steel With Excellent Haz Toughness - Google PatentsSteve OoiNo ratings yet
- Factors Controlling Naphthenic Acid Corrosion: A. Turnbull, E. Slavcheva, and B. ShoneDocument9 pagesFactors Controlling Naphthenic Acid Corrosion: A. Turnbull, E. Slavcheva, and B. ShoneSteve OoiNo ratings yet
- Watson 2009Document11 pagesWatson 2009Steve OoiNo ratings yet
- Characterization of Solutions by PH and EhDocument1 pageCharacterization of Solutions by PH and EhSteve OoiNo ratings yet
- JPH04365841A Original Document 20200926111404 ADocument7 pagesJPH04365841A Original Document 20200926111404 ASteve OoiNo ratings yet
- Stainless Steel 1991Document1 pageStainless Steel 1991Steve OoiNo ratings yet
- JPH06220583A Original Document 20200926110109 ADocument8 pagesJPH06220583A Original Document 20200926110109 ASteve OoiNo ratings yet
- Crusher 4Document39 pagesCrusher 4kediliterapiNo ratings yet
- Daewoo SJ-210H DSJ-6000LHMDocument44 pagesDaewoo SJ-210H DSJ-6000LHMMarco Antonio100% (5)
- CE Laws L8 L15Document470 pagesCE Laws L8 L15Edwin BernatNo ratings yet
- Part 1. Question 1-7. Complete The Notes Below. Write NO MORE THAN THREE WORDS AND/OR A NUMBER For Each AnswerDocument13 pagesPart 1. Question 1-7. Complete The Notes Below. Write NO MORE THAN THREE WORDS AND/OR A NUMBER For Each Answerahmad amdaNo ratings yet
- International Rice Research Newsletter Vol12 No.4Document67 pagesInternational Rice Research Newsletter Vol12 No.4ccquintosNo ratings yet
- 01 C. Toolbar and Message BoxDocument10 pages01 C. Toolbar and Message Boxradu.iacobNo ratings yet
- Theben Timer SUL 181DDocument2 pagesTheben Timer SUL 181DFerdiNo ratings yet
- 2005 Warehouse Benchmark in GR PTDocument59 pages2005 Warehouse Benchmark in GR PTMarco Antonio Oliveira NevesNo ratings yet
- 7 ApportionmentDocument46 pages7 Apportionmentsass sofNo ratings yet
- Interection 2 Reading Teacher's Book PDFDocument165 pagesInterection 2 Reading Teacher's Book PDFتركي الزهراني0% (1)
- Lecture 08Document32 pagesLecture 08SusovanNo ratings yet
- CalculusDocument101 pagesCalculuskusnoNo ratings yet
- CfoDocument13 pagesCfocarmen pirvanNo ratings yet
- Introduction To Regional PlanningDocument27 pagesIntroduction To Regional Planningadeeba siddiquiNo ratings yet
- A Control Method For Power-Assist Devices Using A BLDC Motor For Manual WheelchairsDocument7 pagesA Control Method For Power-Assist Devices Using A BLDC Motor For Manual WheelchairsAhmed ShoeebNo ratings yet
- m07srt Lesson KmarlinkDocument3 pagesm07srt Lesson Kmarlinkapi-515106812No ratings yet
- RCD ManagementDocument6 pagesRCD ManagementPindoterONo ratings yet
- 10 Problem For The Topic 9 & 10 Hicao GroupDocument4 pages10 Problem For The Topic 9 & 10 Hicao GroupArvin ArmojallasNo ratings yet
- A Review On Different Yogas Used in The Management of Mandali Damsa Vrana W.S.R. To KriyakaumudiDocument11 pagesA Review On Different Yogas Used in The Management of Mandali Damsa Vrana W.S.R. To KriyakaumudiTiya TiwariNo ratings yet
- SLHT Grade 7 CSS Week 5 Without Answer KeyDocument6 pagesSLHT Grade 7 CSS Week 5 Without Answer KeyprinceyahweNo ratings yet
- Perfect Picture SummaryDocument3 pagesPerfect Picture SummaryReiaNo ratings yet
- Asme Bladder Accumulator DatasheetDocument3 pagesAsme Bladder Accumulator DatasheetSamad A BakarNo ratings yet
- Financial Analysis of Ashok LeylandDocument120 pagesFinancial Analysis of Ashok LeylandSiva Kumaravel0% (1)
- Grade 7 First Quarter ExamDocument3 pagesGrade 7 First Quarter ExamBILLY JOE ARELLANONo ratings yet
- PLX Model OfficialDocument105 pagesPLX Model OfficialBảo Ngọc LêNo ratings yet
- One God One People February 2013Document297 pagesOne God One People February 2013Stig DragholmNo ratings yet