Professional Documents
Culture Documents
Chap - 28 - New
Uploaded by
Abubakar Siddiq RaminOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chap - 28 - New
Uploaded by
Abubakar Siddiq RaminCopyright:
Available Formats
CHEMISTRY IGCSE NOTES
Chapter – 28
Esters - If we heat alcohols and carboxylic acids together they’ll form an ester. Esters
are a homologous series which contain the functional group ‘-COO’ and can be
identified by their distinctive fruity smell. They also evaporate easily (we say they are
volatile) which makes them a useful group of compounds to use in perfumes and food
flavourings.
Let’s look at the reaction between ethanol and ethanoic acid to produce the ester, ethyl
ethanoate. This reaction requires an acid catalyst, which is usually concentrated sulfuric
acid.
CH3COOH + CH3CH2OH → CH3COOCH2CH3 + H2O
Naming esters - The first part of the name comes from the alcohol and the second part
from the carboxylic acid. For example, the ester ethyl propanoate would have been
formed by reacting ethanol and propanoic acid together.
You might also like
- Practice Makes Perfect in Chemistry: Organic ChemistryFrom EverandPractice Makes Perfect in Chemistry: Organic ChemistryRating: 3 out of 5 stars3/5 (1)
- ESTERS 3nd FixedDocument6 pagesESTERS 3nd Fixed陈洁No ratings yet
- Practice Makes Perfect in Chemistry: Organic Chemistry with AnswersFrom EverandPractice Makes Perfect in Chemistry: Organic Chemistry with AnswersNo ratings yet
- CH3 Alcohols, Carboxylic Acids and EstersDocument8 pagesCH3 Alcohols, Carboxylic Acids and EstersDoc CrocNo ratings yet
- Esters Fats and Oils Pupil NotesDocument19 pagesEsters Fats and Oils Pupil NotesMasumNo ratings yet
- IGCSE Chemistry Notes: Organic CompoundsDocument7 pagesIGCSE Chemistry Notes: Organic CompoundsFasih AhmadNo ratings yet
- Synthesis of Methyl SalicylateDocument2 pagesSynthesis of Methyl SalicylateMich Tolentino0% (1)
- Tyndale-Biscoe and Mallinson Society: Senior DepartmentDocument11 pagesTyndale-Biscoe and Mallinson Society: Senior DepartmentAlima IqbalNo ratings yet
- 487 - Concept, Sources, Nomenclature and Isomerism in Alkanoate (Esters) .Document4 pages487 - Concept, Sources, Nomenclature and Isomerism in Alkanoate (Esters) .emmanuelirem805No ratings yet
- Bautista, John Mhar M. (Experiment 7)Document4 pagesBautista, John Mhar M. (Experiment 7)2g8vdspqm5No ratings yet
- ESTERSDocument36 pagesESTERSAbdihakemNo ratings yet
- Alcohols and Carboxylic Acids GuideDocument5 pagesAlcohols and Carboxylic Acids GuideM.zuhair asifNo ratings yet
- Ester FormationDocument6 pagesEster Formationkriss WongNo ratings yet
- Unit 11 - Alcohols, Phenols and EthersDocument41 pagesUnit 11 - Alcohols, Phenols and EthersPoovaraahan RaghuveeranNo ratings yet
- EsterDocument2 pagesEsterCyrisse MONTANONo ratings yet
- Rio Tinto Rössing Foundation Grade 11-12 Chemistry Notes on Functional Groups & EstersDocument3 pagesRio Tinto Rössing Foundation Grade 11-12 Chemistry Notes on Functional Groups & EstersBradleyNo ratings yet
- CHAPTER 9 HydrocarbonsDocument2 pagesCHAPTER 9 HydrocarbonsJaishree RamNo ratings yet
- Functional Groups: Naming of EstersDocument6 pagesFunctional Groups: Naming of EsterspappadakunduNo ratings yet
- Chemistry NoteDocument14 pagesChemistry NotePreet JAYSWALNo ratings yet
- ALKANOLSDocument4 pagesALKANOLSPrinceblesed EdemNo ratings yet
- Structure & Properties of Alcohols & EthersDocument4 pagesStructure & Properties of Alcohols & EthersAlelli Bianca Daniel AlipioNo ratings yet
- Kimia OrganikDocument7 pagesKimia OrganikZahra AlfinaNo ratings yet
- Table of Content: Organic Synthesis: Formation of An Ester Lab ReportDocument11 pagesTable of Content: Organic Synthesis: Formation of An Ester Lab ReportMuhammad Irfan Malik100% (1)
- Try To Answer: IsopropylbenzeneDocument33 pagesTry To Answer: IsopropylbenzeneRoxanne Sioco100% (1)
- Naming EthersDocument11 pagesNaming EthersPedro SuyuNo ratings yet
- LabReport Experiment#5 ParthPatel Copy 2Document7 pagesLabReport Experiment#5 ParthPatel Copy 2parthNo ratings yet
- ALKANOATEDocument2 pagesALKANOATEpeelenguyNo ratings yet
- 5 Hydrocarbon Derivatives 2Document28 pages5 Hydrocarbon Derivatives 2Marivic TayabanNo ratings yet
- CHEMDocument24 pagesCHEMbuenaventurasheelohNo ratings yet
- Alchohol and EterDocument12 pagesAlchohol and EterFaza Afina RizaNo ratings yet
- 17.5 To 17.8 Organic Chemistry Notes-2Document7 pages17.5 To 17.8 Organic Chemistry Notes-2ahmedNo ratings yet
- Chemistry Module 9Document4 pagesChemistry Module 9angelo aquinoNo ratings yet
- Sir Gero MOdule 8Document3 pagesSir Gero MOdule 8angelo aquinoNo ratings yet
- Esters PresentationDocument9 pagesEsters PresentationClarissa100% (2)
- Module 3-Carbon CompoundsDocument57 pagesModule 3-Carbon CompoundsJaynarose Castillo Rivera100% (5)
- ESTERS: Derivatives of Carboxylic AcidsDocument13 pagesESTERS: Derivatives of Carboxylic AcidsMohd NazriNo ratings yet
- Note JJ JJDocument23 pagesNote JJ JJms9216974No ratings yet
- Module 4 - Alcohol, Ether and AldehydeDocument62 pagesModule 4 - Alcohol, Ether and AldehydePrincess NavarroNo ratings yet
- Organic Chemistry Concepts ExplainedDocument9 pagesOrganic Chemistry Concepts ExplainedM.zuhair asifNo ratings yet
- Science Ester Info 1Document10 pagesScience Ester Info 1Albert AntonioNo ratings yet
- 7-10.4carboxylic Acids and EstersDocument10 pages7-10.4carboxylic Acids and EsterstakomolyentinNo ratings yet
- Igcse Separate Sciences Topic C14: Organic Chemistry Revision NotesDocument9 pagesIgcse Separate Sciences Topic C14: Organic Chemistry Revision NotesJamiu Yusuf AsukuNo ratings yet
- EthersDocument11 pagesEthersbomzanaakritiNo ratings yet
- Asm 52316Document7 pagesAsm 52316krishna.madhan2009No ratings yet
- Chapter 12 Saturated HydrocarbonsDocument17 pagesChapter 12 Saturated HydrocarbonsChristian Guimmayen ArizoNo ratings yet
- Organic ChemistryDocument13 pagesOrganic ChemistryKC De leonNo ratings yet
- Laporan IGF AlimDocument11 pagesLaporan IGF Alimppg.risdaniar99130No ratings yet
- The Saturated Hydrocarbons: Alkanes and Cycloalkanes: Contrasts Between Organic and Inorganic MoleculesDocument9 pagesThe Saturated Hydrocarbons: Alkanes and Cycloalkanes: Contrasts Between Organic and Inorganic MoleculesNaveenNo ratings yet
- Organic Chemistry Basics: Hydrocarbons, Alcohols & AcidsDocument15 pagesOrganic Chemistry Basics: Hydrocarbons, Alcohols & AcidsEnica RichardNo ratings yet
- Cita High School, Port Harcourt Subject: Chemistry Class: Ss3 Week TopicDocument107 pagesCita High School, Port Harcourt Subject: Chemistry Class: Ss3 Week TopicYushau Muhammad LawalNo ratings yet
- Isomerism in Alkanoic AcidsDocument6 pagesIsomerism in Alkanoic AcidsnelsonNo ratings yet
- Organic Compound - Part IIDocument4 pagesOrganic Compound - Part IITherese ClaireNo ratings yet
- B. Physical Properties of AlcoholsDocument4 pagesB. Physical Properties of AlcoholsAnjo Pasiolco CanicosaNo ratings yet
- Chem Notes No6Document31 pagesChem Notes No6AnyhaNo ratings yet
- Alcohols Phenols and Ether by AarkumarDocument0 pagesAlcohols Phenols and Ether by AarkumarNikhil Surya MukhiNo ratings yet
- Organic Chemistry CurrentDocument48 pagesOrganic Chemistry CurrentBierzo JomarNo ratings yet
- EsterificareaDocument43 pagesEsterificareaMahagney SalehNo ratings yet
- ESTERSDocument37 pagesESTERSFirdausia Rahma PutriNo ratings yet
- Genetic Modification: DNA A, T, G, and CDocument4 pagesGenetic Modification: DNA A, T, G, and CAbubakar Siddiq RaminNo ratings yet
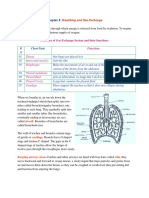
- Breathing and Gas Exchange: RespirationDocument4 pagesBreathing and Gas Exchange: RespirationAbubakar Siddiq RaminNo ratings yet
- Natural Selection and Evolution EvolutionDocument2 pagesNatural Selection and Evolution EvolutionAbubakar Siddiq RaminNo ratings yet
- Chemistry Igcse Notes - 26 AlcoholDocument6 pagesChemistry Igcse Notes - 26 AlcoholAbubakar Siddiq Ramin100% (1)
- Unit 1 AnswersDocument21 pagesUnit 1 AnswersAhnaf ChaudhuryNo ratings yet
- Mathematics B: Edexcel IGCSEDocument20 pagesMathematics B: Edexcel IGCSEsohaibNo ratings yet
- Biology AnswersDocument20 pagesBiology AnswersMashrur AreebNo ratings yet
- Chap - 29 - NewDocument4 pagesChap - 29 - NewAbubakar Siddiq RaminNo ratings yet
- Model Question 01 - Section B - Letter WritingDocument2 pagesModel Question 01 - Section B - Letter WritingAbubakar Siddiq RaminNo ratings yet
- My Time Is NowDocument1 pageMy Time Is NowAbubakar Siddiq RaminNo ratings yet
- Model Question - 01 (Text)Document3 pagesModel Question - 01 (Text)Abubakar Siddiq RaminNo ratings yet
- 4PM0 02 Que 20170123Document36 pages4PM0 02 Que 20170123Eldric WhiteNo ratings yet
- 1BI0 1H June18 QP-GCSE-Edexcel-BiologyDocument36 pages1BI0 1H June18 QP-GCSE-Edexcel-BiologyRabia RafiqueNo ratings yet
- Alem's Culture Shock in LondonDocument14 pagesAlem's Culture Shock in LondonAbubakar Siddiq RaminNo ratings yet
- Chapter 1Document9 pagesChapter 1Abubakar Siddiq RaminNo ratings yet
- 4PH1 2PR Rms 20190822Document14 pages4PH1 2PR Rms 20190822Åzmâñ Khäñ100% (3)
- Markscheme Paper2 January2018Document20 pagesMarkscheme Paper2 January2018ARian Araf RahmanNo ratings yet
- M3 2015 PDFDocument18 pagesM3 2015 PDFSunnyNo ratings yet