Professional Documents
Culture Documents
Chemistry Igcse Notes - 26 Alcohol
Uploaded by
Abubakar Siddiq Ramin100%(1)100% found this document useful (1 vote)
77 views6 pagesBiology
Original Title
Chap - 26 - new
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentBiology
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
100%(1)100% found this document useful (1 vote)
77 views6 pagesChemistry Igcse Notes - 26 Alcohol
Uploaded by
Abubakar Siddiq RaminBiology
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 6
CHEMISTRY IGCSE NOTES
Chapter – 26
Alcohol – All alcohols contain an OH group
covalently bonded to a carbon chain. The
most common alcohol is ethanol. Displayed
formulas of the members of the alcohol
group are shown below:
Oxidation of ethanol:
When you heat
ethanol with
potassium dichromate
under reflux in the
presence of dilute sulfuric acid which acts as
a catalyst, the dichromate acts as an
oxidising agent and oxidises ethanol to form
ethanoic acid. We observe that the orange
dichromate solution has turned green due to
the presence of Cr3+ ions.
Manufacture of ethanol by:
Fermentation – We will add yeast to a
sugar or starch solution and leave it in 30° –
40°C temperature for several days in the
absence of air. Enzymes in the yeast
(zymase) will convert the sugar into ethanol
and carbon dioxide.
The sucrose (sugar) is split into two smaller
sugars – glucose and fructose. Glucose and
fructose have the same molecular formula,
but different structures. They are isomers.
Enzymes in the yeast convert these sugars
into ethanol and carbon dioxide in a
multitude of small steps. Yeast is killed by
more than 15% of ethanol in the mixture, so
it is purified by fractional distillation.
C12H22O11 (aq) + H2O (l) → C6H12O6 (aq) + C6H12O6 (aq)
C6H12O6 (aq) → 2C2H5OH (aq) + 2CO2 (g)
Hydration of ethene – Ethanol is also made
by reacting ethene with steam, which is
called hydration. The process details are
included in the previous chapter.
C2H4 (g) + H2O (g) → C2H5OH (g)
Comparison between the methods of ethanol production:
Fermentation Hydration of ethene
Use of Uses Uses non-
resources renewable renewable
resources like resources like
sugar cane oil
Type of An inefficient An more
process batch process efficient
continuous
flow process
Rate of slow rapid
reaction
Quality of Produces very Produces
product impure much purer
ethanol that ethanol
requires
further
processing
Reaction Uses gentle Uses high
conditions temperature temperature
and ordinary and pressure
pressure
Dehydration of ethanol - Dehydration is
the removal of water from a compound. We
will soak a mineral wool in ethanol and
place it at the end of a test tube containing
hot aluminium oxide acting as a catalyst.
Ethanol vapour will pass over hot
aluminium oxide. Ethene will be collected
over water. Other alcohols too dehydrate in
a similar way.
C2H5OH (g) → C2H4 (g) + H2O (l)
You might also like
- Compiled Reviewer For Midterm ExamDocument12 pagesCompiled Reviewer For Midterm ExamMelony Dela fuenteNo ratings yet
- Tutorial 3Document3 pagesTutorial 3CHANDREN ARUMUGAM0% (1)
- Cambridge Checkpoint Science Workbook 2 AnswersDocument33 pagesCambridge Checkpoint Science Workbook 2 AnswersmarryNo ratings yet
- Year 8 PhotosynthesisDocument9 pagesYear 8 Photosynthesisbali yunNo ratings yet
- Solubility InvestigationDocument3 pagesSolubility InvestigationNoaNo ratings yet
- Fle1 WB IssuuDocument53 pagesFle1 WB Issuumohammedshiraz1608No ratings yet
- DHA Education System: Book List (2020-21)Document1 pageDHA Education System: Book List (2020-21)Syed Abdul Ahad TirmidhiNo ratings yet
- Wesley YEAR 7 BooklistDocument2 pagesWesley YEAR 7 BooklistLeeHoai Chai100% (1)
- 0893 Lower Secondary Science Stage 9 Scheme of Work Tcm143 595697Document108 pages0893 Lower Secondary Science Stage 9 Scheme of Work Tcm143 595697ainurrohmahekahappysusantiworkNo ratings yet
- H.W Yr.7Document7 pagesH.W Yr.7abdelrahmanelmadany11No ratings yet
- G7 Respiration Revision 1Document7 pagesG7 Respiration Revision 1PRIYA100% (1)
- International Lower Secondary Science Workbook 3Document15 pagesInternational Lower Secondary Science Workbook 3Just SpawnNo ratings yet
- Cambridge Lower Secondary Mathematics Workbook 8 9781108746403book-8-10Document3 pagesCambridge Lower Secondary Mathematics Workbook 8 9781108746403book-8-10aloonaothman0% (1)
- Unit 7 The Ant and The Beetle New Vocab.: Cairo GovernorateDocument60 pagesUnit 7 The Ant and The Beetle New Vocab.: Cairo GovernorateHanineNo ratings yet
- Secondary Progression Test Stage 8 English Paper 1Document12 pagesSecondary Progression Test Stage 8 English Paper 1martajambalaoNo ratings yet
- Cell Structure and Function - Magnification CalculationDocument17 pagesCell Structure and Function - Magnification CalculationHamza KhanNo ratings yet
- First Term Science Year 5 Yearly Plan Themes A: Investigating Living ThingsDocument24 pagesFirst Term Science Year 5 Yearly Plan Themes A: Investigating Living ThingsXgeniusXNo ratings yet
- Solved Collected Worksheets Science 5Document30 pagesSolved Collected Worksheets Science 5Yazeed MohmmadNo ratings yet
- 9701 s05 QP 4Document12 pages9701 s05 QP 4Hubbak KhanNo ratings yet
- Checkpoint Task Photosynthesis: Instructions and Answers For TeachersDocument17 pagesCheckpoint Task Photosynthesis: Instructions and Answers For TeachersjeanNo ratings yet
- Gr-6 t2 Science SQP Ay2022-23Document10 pagesGr-6 t2 Science SQP Ay2022-23bjain21No ratings yet
- Oct 2020 p1 QP 1113Document20 pagesOct 2020 p1 QP 1113Lee Jia Bao BerniceNo ratings yet
- Physics Syllabus 2026-2028Document63 pagesPhysics Syllabus 2026-2028jeaninealemamensahNo ratings yet
- Notes PDFDocument38 pagesNotes PDFAbigail SachNo ratings yet
- Protein Synthesis Story Project Pap 2015Document4 pagesProtein Synthesis Story Project Pap 2015Mark Jemuel Cacanando SalcedoNo ratings yet
- Workbook Unit1 AnswersDocument2 pagesWorkbook Unit1 AnswersIce KingNo ratings yet
- Chapter 2: Cell As The Basic Unit of Life: Science Module Form 1 - Chapter 2Document13 pagesChapter 2: Cell As The Basic Unit of Life: Science Module Form 1 - Chapter 2Thiba KrishnanNo ratings yet
- Physics Project - Sources of EnergyDocument16 pagesPhysics Project - Sources of Energyyasiero75% (4)
- Characteristics-and-classification-of-living-organisms-Paper 2 Classified Solved Question CIE IGCSE GCSE OLevel BiologyDocument32 pagesCharacteristics-and-classification-of-living-organisms-Paper 2 Classified Solved Question CIE IGCSE GCSE OLevel BiologyIGCSE Physics & ChemistryNo ratings yet
- EDU 44277 IGCSE Science WYNTK Digital 2020 PDFDocument2 pagesEDU 44277 IGCSE Science WYNTK Digital 2020 PDFsana adeel0% (2)
- Checkpoint Science Challenge 7 - Part 01Document9 pagesCheckpoint Science Challenge 7 - Part 01KhNo ratings yet
- Lower Secondary Science ELS Workbook 7-AnswersDocument4 pagesLower Secondary Science ELS Workbook 7-AnswersDr. Sai Ko Ko ZawNo ratings yet
- Cambridge Primary Science Year 7 WB 2nd Edition PDF Chemical Compounds Chlorine 5Document1 pageCambridge Primary Science Year 7 WB 2nd Edition PDF Chemical Compounds Chlorine 5Rafih AhmadNo ratings yet
- Cambridge Primary Science Challenge 6Document15 pagesCambridge Primary Science Challenge 6Sara 3amer100% (1)
- Price List: Oxford University Press School and College Textbooks: Effective 1 JanuaDocument4 pagesPrice List: Oxford University Press School and College Textbooks: Effective 1 JanuaFayaz AhmedNo ratings yet
- Topic 8 Transport in PlantsDocument45 pagesTopic 8 Transport in PlantsCOXMIC FNNo ratings yet
- Long-Term Planning Template: Cambridge Lower Secondary Science Stage 8Document3 pagesLong-Term Planning Template: Cambridge Lower Secondary Science Stage 8marycperez100% (1)
- Year 8 Science Scheme of WorkDocument90 pagesYear 8 Science Scheme of WorkMimiNo ratings yet
- Mce Igcse Chemistry PPT c02Document27 pagesMce Igcse Chemistry PPT c02Lim Khee HanNo ratings yet
- Unit of Study PhotosynthesisDocument18 pagesUnit of Study PhotosynthesisNaurah Khairiyah RumakatNo ratings yet
- Coursebook Answers: Science in Context: Lord of The RingsDocument39 pagesCoursebook Answers: Science in Context: Lord of The RingsEshanNo ratings yet
- Cambridge Lower Secondary Mathematics Workbook 8 9781108746403book-7-8Document2 pagesCambridge Lower Secondary Mathematics Workbook 8 9781108746403book-7-8aloonaothman100% (1)
- Checkpoint Science Revision GuideDocument252 pagesCheckpoint Science Revision Guidemixiaoquan98No ratings yet
- Final SVKM Quot 21.08.2019Document1 pageFinal SVKM Quot 21.08.2019aliispowerNo ratings yet
- S9 Unit 1 WorksheetsDocument10 pagesS9 Unit 1 WorksheetsLobna ShabanNo ratings yet
- Science Workbook 2 AnswersDocument29 pagesScience Workbook 2 AnswersabbrishhNo ratings yet
- Free Cambridge Checkpoint Science Workbook 8 PDFDocument5 pagesFree Cambridge Checkpoint Science Workbook 8 PDFWin Myat ThuzarNo ratings yet
- Cambridge Primary English Workbook 6 - 9781108746281 - WB6 - FLE - SAMPLEDocument205 pagesCambridge Primary English Workbook 6 - 9781108746281 - WB6 - FLE - SAMPLEAya Ahmed QotbNo ratings yet
- Prim Maths 5 2ed TR Aditional Teaching Ideas 1Document6 pagesPrim Maths 5 2ed TR Aditional Teaching Ideas 1Andrew Amankwah100% (1)
- IGCSE Coordinated Sciences Biological MoleculesDocument9 pagesIGCSE Coordinated Sciences Biological MoleculesSeonaid McDonaldNo ratings yet
- Cambridge Primary Science Scheme of Work Learning Objectives Stage 2Document4 pagesCambridge Primary Science Scheme of Work Learning Objectives Stage 2Carolyne AchiengNo ratings yet
- Checkpoint ScienceDocument8 pagesCheckpoint ScienceNiyi OmodaraNo ratings yet
- English Worksheet Grade 4Document5 pagesEnglish Worksheet Grade 4Samreen SoomroNo ratings yet
- Primary Maths 2 StudentBook 2Document134 pagesPrimary Maths 2 StudentBook 2Ahmet BabacNo ratings yet
- G6 Unit 6 English WB NotesDocument2 pagesG6 Unit 6 English WB NotesIshaan BhoirNo ratings yet
- Cambridge Lower Secondary Science Learners Book 8 - Cambridge GO 2Document1 pageCambridge Lower Secondary Science Learners Book 8 - Cambridge GO 27dwg75s8mgNo ratings yet
- Contentdamone Dot Comone Dot Cominternational Schoolspdfsilower Secondaryinspire English InternatDocument24 pagesContentdamone Dot Comone Dot Cominternational Schoolspdfsilower Secondaryinspire English InternatErny MasiloNo ratings yet
- Cambridge - Maths - LB2 - COVERDocument1 pageCambridge - Maths - LB2 - COVERsabbar designNo ratings yet
- Answers To Core Revision Exercises: Data Handling: Worksheet 4: Collecting, Organising and Displaying DataDocument3 pagesAnswers To Core Revision Exercises: Data Handling: Worksheet 4: Collecting, Organising and Displaying Datamk hat100% (1)
- ALCOHOLS (Class)Document9 pagesALCOHOLS (Class)adritaNo ratings yet
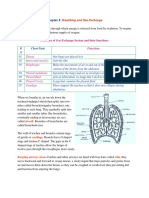
- Breathing and Gas Exchange: RespirationDocument4 pagesBreathing and Gas Exchange: RespirationAbubakar Siddiq RaminNo ratings yet
- Natural Selection and Evolution EvolutionDocument2 pagesNatural Selection and Evolution EvolutionAbubakar Siddiq RaminNo ratings yet
- Chap - 29 - NewDocument4 pagesChap - 29 - NewAbubakar Siddiq RaminNo ratings yet
- Unit 1 AnswersDocument21 pagesUnit 1 AnswersAhnaf ChaudhuryNo ratings yet
- Genetic Modification: DNA A, T, G, and CDocument4 pagesGenetic Modification: DNA A, T, G, and CAbubakar Siddiq RaminNo ratings yet
- Biology AnswersDocument20 pagesBiology AnswersMashrur AreebNo ratings yet
- Chap - 28 - NewDocument1 pageChap - 28 - NewAbubakar Siddiq RaminNo ratings yet
- My Time Is NowDocument1 pageMy Time Is NowAbubakar Siddiq RaminNo ratings yet
- Model Question - 01Document14 pagesModel Question - 01Abubakar Siddiq RaminNo ratings yet
- Model Question 01 - Section B - Letter WritingDocument2 pagesModel Question 01 - Section B - Letter WritingAbubakar Siddiq RaminNo ratings yet
- Mathematics B: Edexcel IGCSEDocument20 pagesMathematics B: Edexcel IGCSEsohaibNo ratings yet
- 1BI0 1H June18 QP-GCSE-Edexcel-BiologyDocument36 pages1BI0 1H June18 QP-GCSE-Edexcel-BiologyRabia RafiqueNo ratings yet
- Model Question - 01 (Text)Document3 pagesModel Question - 01 (Text)Abubakar Siddiq RaminNo ratings yet
- Chapter 1Document9 pagesChapter 1Abubakar Siddiq RaminNo ratings yet
- M3 2015 PDFDocument18 pagesM3 2015 PDFSunnyNo ratings yet
- 4PH1 2PR Rms 20190822Document14 pages4PH1 2PR Rms 20190822Åzmâñ Khäñ100% (3)
- Markscheme Paper2 January2018Document20 pagesMarkscheme Paper2 January2018ARian Araf RahmanNo ratings yet
- 4PM0 02 Que 20170123Document36 pages4PM0 02 Que 20170123Eldric WhiteNo ratings yet
- UV LED ReviewDocument27 pagesUV LED ReviewDharshini DeviNo ratings yet
- Food LabellingDocument27 pagesFood LabellingOsman AliNo ratings yet
- CBLM FloresDocument42 pagesCBLM FloresJinky Aydalla100% (2)
- The Permaculture Home Garden (PDFDrive)Document194 pagesThe Permaculture Home Garden (PDFDrive)Gigi Franco100% (2)
- MushWorld - Oyster Mushroom Cultivation Handbook-MushWorld (2004)Document287 pagesMushWorld - Oyster Mushroom Cultivation Handbook-MushWorld (2004)Il anonimoyyy100% (1)
- Esterifikasyon Reaktor Onemli2Document20 pagesEsterifikasyon Reaktor Onemli2Mehmet AydinNo ratings yet
- Degradation of Acid Cyanide Poison in Rubber Seed (Hevea Brasiliensis) After Treatment With Rice Husk AshDocument3 pagesDegradation of Acid Cyanide Poison in Rubber Seed (Hevea Brasiliensis) After Treatment With Rice Husk AshAyulia AnnisaNo ratings yet
- 2022.03 Elevators enDocument4 pages2022.03 Elevators enrajksharmaNo ratings yet
- Agriculture, Ecosystems and Environment: ReviewDocument12 pagesAgriculture, Ecosystems and Environment: ReviewFernanda GonsalesNo ratings yet
- AIR PRODUCTS Cleaning Formulary BrochureDocument56 pagesAIR PRODUCTS Cleaning Formulary BrochureZoltán Király100% (6)
- Freezing of Bread PublishedDocument6 pagesFreezing of Bread PublishedshashlikNo ratings yet
- Biology 11: Kingdom Plantae: Unit TopicsDocument18 pagesBiology 11: Kingdom Plantae: Unit TopicsMariel AnaNo ratings yet
- Cambridge IGCSE: Biology 0610/23Document16 pagesCambridge IGCSE: Biology 0610/23YashodhaNo ratings yet
- CBSE Sample Paper Class 12 Chemistry Set 5 PDFDocument4 pagesCBSE Sample Paper Class 12 Chemistry Set 5 PDFSidharth SabharwalNo ratings yet
- Article Sample IOPDocument9 pagesArticle Sample IOPGilang BuditamaNo ratings yet
- Cho 393 Sem ReadyDocument11 pagesCho 393 Sem Readypapey42271No ratings yet
- Presented To The Academe Of: A Research ProposalDocument33 pagesPresented To The Academe Of: A Research ProposalTae-tae LachimolalaNo ratings yet
- 18 SWLB Part 1Document14 pages18 SWLB Part 1Ronald BaguisNo ratings yet
- Unit 5 BK Academic Connections 1 1Document20 pagesUnit 5 BK Academic Connections 1 1api-515535366No ratings yet
- 9b PlantsDocument2 pages9b PlantsOneth RajapakseNo ratings yet
- Hi-Tech Magazine Jun 2022 - How To Start Manufacturing Industry - Project Consultancy ServicesDocument42 pagesHi-Tech Magazine Jun 2022 - How To Start Manufacturing Industry - Project Consultancy ServicesSachin SharmaNo ratings yet
- White Truffle NA21983 CR D 4Document2 pagesWhite Truffle NA21983 CR D 4PPIC sinergiNo ratings yet
- M.Tech. (Full Time) - Food Safety & Quality Management Curriculum & Syllabus 2015 - 2016Document36 pagesM.Tech. (Full Time) - Food Safety & Quality Management Curriculum & Syllabus 2015 - 2016Anwara KhatunNo ratings yet
- Alcohol ProductionDocument11 pagesAlcohol ProductionRabwa RazakNo ratings yet
- 1 PBDocument48 pages1 PBRachmattullahNo ratings yet
- 8 - Edible Landscape DesignDocument66 pages8 - Edible Landscape DesignMehmet EvliyaNo ratings yet
- Letter To Agri Research DirectorDocument3 pagesLetter To Agri Research DirectorChiku Amani ChikotiNo ratings yet
- (Poly, Many Mer, Unit) Monomers (Mono, One) : Carbon (C), Hydrogen (H), Oxygen (O) and Nitrogen (N) PolymersDocument5 pages(Poly, Many Mer, Unit) Monomers (Mono, One) : Carbon (C), Hydrogen (H), Oxygen (O) and Nitrogen (N) PolymersDaneth Julia TuberaNo ratings yet
- Acknowledgement: Department of Chemical Engineering (Food Stream)Document88 pagesAcknowledgement: Department of Chemical Engineering (Food Stream)Gemechis TolaNo ratings yet
- Surface Water PFAS StrategyDocument38 pagesSurface Water PFAS StrategyBea ParrillaaNo ratings yet