Professional Documents
Culture Documents
Ionization Energy Trends
Uploaded by
Oluwabamire OreoluwaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ionization Energy Trends
Uploaded by
Oluwabamire OreoluwaCopyright:
Available Formats
Updated July 03, 2019
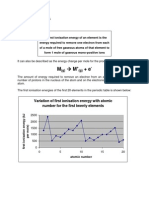
Ionization energy is the energy required to remove an electron from
a gaseous atom or ion. The first or initial ionization energy or Ei of an atom
or molecule is the energy required to remove one mole of electrons from one
mole of isolated gaseous atoms or ions.
You may think of ionization energy as a measure of the difficulty of removing
electron or the strength by which an electron is bound. The higher the ionization
energy, the more difficult it is to remove an electron. Therefore, ionization energy
is in indicator of reactivity. Ionization energy is important because it can be used
to help predict the strength of chemical bonds.
Also Known As: ionization potential, IE, IP, ΔH°
Units: Ionization energy is reported in units of kilojoule per mole (kJ/mol) or
electron volts (eV).
Ionization Energy Trend in the Periodic Table
Ionization, together with atomic and ionic radius, electronegativity, electron
affinity, and metallicity, follows a trend on the periodic table of elements.
Ionization energy generally increases moving from left to right across an
element period (row). This is because the atomic radius generally decreases
moving across a period, so there is a greater effective attraction between
the negatively charged electrons and positively-charged nucleus. Ionization
is at its minimum value for the alkali metal on the left side of the table and
a maximum for the noble gas on the far right side of a period. The noble
gas has a filled valence shell, so it resists electron removal.
Ionization decreases moving top to bottom down an element group
(column). This is because the principal quantum number of the outermost
electron increases moving down a group. There are more protons in atoms
moving down a group (greater positive charge), yet the effect is to pull in
the electron shells, making them smaller and screening outer electrons
from the attractive force of the nucleus. More electron shells are added
moving down a group, so the outermost electron becomes increasingly
distance from the nucleus.
First, Second, and Subsequent Ionization Energies
The energy required to remove the outermost valence electron from a neutral
atom is the first ionization energy. The second ionization energy is that required
to remove the next electron, and so on. The second ionization energy is always
higher than the first ionization energy. Take, for example, an alkali metal atom.
Removing the first electron is relatively easy because its loss gives the atom a
stable electron shell. Removing the second electron involves a new electron shell
that is closer and more tightly bound to the atomic nucleus.
The first ionization energy of hydrogen may be represented by the following
equation:
H(g) → H+(g) + e-
ΔH° = -1312.0 kJ/mol
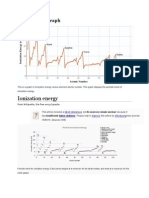
Exceptions to the Ionization Energy Trend
If you look at a chart of first ionization energies, two exceptions to the trend are
readily apparent. The first ionization energy of boron is less than that of
beryllium and the first ionization energy of oxygen is less than that of nitrogen.
The reason for the discrepancy is due to the electron configuration of these
elements and Hund's rule. For beryllium, the first ionization potential electron
comes from the 2s orbital, although ionization of boron involves a 2p electron.
For both nitrogen and oxygen, the electron comes from the 2p orbital, but the
spin is the same for all 2p nitrogen electrons, while there is a set of paired
electrons in one of the 2p oxygen orbitals.
Key Points
Ionization energy is the minimum energy required to remove an electron
from an atom or ion in the gas phase.
The most common units of ionization energy are kilojoules per mole
(kJ/M) or electron volts (eV).
Ionization energy exhibits periodicity on the periodic table.
The general trend is for ionization energy to increase moving from left to
right across an element period. Moving left to right across a period, atomic
radius decreases, so electrons are more attracted to the (closer) nucleus.
The general trend is for ionization energy to decrease moving from top to
bottom down a periodic table group. Moving down a group, a valence shell
is added. The outermost electrons are further from the positive-charged
nucleus, so they are easier to remove.
You might also like
- Chemical Classification and Periodic TrendsDocument20 pagesChemical Classification and Periodic TrendsRAJEEV KARAKOTINo ratings yet
- Rigid Block Foundation Design for Rotating EquipmentDocument7 pagesRigid Block Foundation Design for Rotating EquipmentApratim DasguptaNo ratings yet
- DRSMARTANDANUOLUWAI NITIATIVE BIO102QUESTIONSANDANSWERSDocument10 pagesDRSMARTANDANUOLUWAI NITIATIVE BIO102QUESTIONSANDANSWERSOluwabamire Oreoluwa100% (8)
- Ionization Energy and Electron AffinityDocument9 pagesIonization Energy and Electron AffinityKhan AaghaNo ratings yet
- Advanced PhysicsDocument374 pagesAdvanced Physicsjoseph moriarty100% (3)
- A Level Chemistry GuideDocument34 pagesA Level Chemistry GuideRexton Jarvis100% (1)
- Ionization EnergyDocument69 pagesIonization EnergyVisalakshi Venkat100% (2)
- Bio101 by Einstein-1Document8 pagesBio101 by Einstein-1Oluwabamire OreoluwaNo ratings yet
- Periodic TrendsDocument9 pagesPeriodic TrendsSobia KashifNo ratings yet
- Periodic Properties of ElementsDocument53 pagesPeriodic Properties of Elementschandro57No ratings yet
- IONISATION ENERGY PERIODIC TRENDDocument12 pagesIONISATION ENERGY PERIODIC TRENDhahajing1980No ratings yet
- Physics Unit PlansDocument85 pagesPhysics Unit PlansGajendra100% (5)
- Marine Hydrodynamics Lectures Week 40Document38 pagesMarine Hydrodynamics Lectures Week 40AntonioNo ratings yet
- Patterns of First Ionisation Energies in The Periodic TableDocument7 pagesPatterns of First Ionisation Energies in The Periodic TableRugen RajNo ratings yet
- Module 1 Part 1 Engine DynamicsDocument57 pagesModule 1 Part 1 Engine DynamicsTara GerdingNo ratings yet
- Cbse Test Paper-01 CLASS - X Science (Electricity and Its Effects)Document5 pagesCbse Test Paper-01 CLASS - X Science (Electricity and Its Effects)krishnareddy_chintalaNo ratings yet
- Periodic TrendsDocument11 pagesPeriodic TrendsFern HofileñaNo ratings yet
- What is a Mass SpectrometerDocument11 pagesWhat is a Mass SpectrometerFiliz Kocayazgan ShanablehNo ratings yet
- Classification of ElementsDocument74 pagesClassification of ElementsJimit Patel BankNo ratings yet
- Ionisation Energy ClassDocument13 pagesIonisation Energy ClassKordell McShineNo ratings yet
- Chapter 3 NotesDocument7 pagesChapter 3 NotesNickNo ratings yet
- Ionization EnergyDocument2 pagesIonization EnergynapilgabethNo ratings yet
- Ion EnergiesDocument4 pagesIon EnergiesNamratha NairNo ratings yet
- PeriodicityDocument6 pagesPeriodicityNetkoNo ratings yet
- Periodic Properties Copy 2Document30 pagesPeriodic Properties Copy 2Rodayna HosniNo ratings yet
- Module - Part 1Document3 pagesModule - Part 1Florence Dela Cruz DolorNo ratings yet
- Electron Configuration and Periodic PropertiesDocument48 pagesElectron Configuration and Periodic Propertiesahmad batataNo ratings yet
- Periodicity of PropertiesDocument5 pagesPeriodicity of PropertiesHaYem AlnNo ratings yet
- Engineering Chemistry Notes UNIT 4Document9 pagesEngineering Chemistry Notes UNIT 4Nivetha ENo ratings yet
- Periodic Trends and The Octet RuleDocument5 pagesPeriodic Trends and The Octet Rule10chirag10No ratings yet
- Periodic Properties of The Elements 608817Document3 pagesPeriodic Properties of The Elements 608817Kumar nayakNo ratings yet
- Electrons in Atoms.Document62 pagesElectrons in Atoms.Md.Tanjim reza TurjoNo ratings yet
- Pdf-1 Periodi Table and Their Properties 20.02.2021Document28 pagesPdf-1 Periodi Table and Their Properties 20.02.2021Himanshu SinghNo ratings yet
- Element and Their Property UsesDocument2 pagesElement and Their Property UsesMa Anna HiyanNo ratings yet
- Periodic Properties and Their Graduation in Group andDocument14 pagesPeriodic Properties and Their Graduation in Group andbhogalsahib1408No ratings yet
- Classification of Elements and Periodicity in PropertiesDocument32 pagesClassification of Elements and Periodicity in PropertiesSanjay DubeyNo ratings yet
- Periodic Trends Resource 1Document8 pagesPeriodic Trends Resource 1anitNo ratings yet
- Periodic Trends: ElectronegativityDocument2 pagesPeriodic Trends: ElectronegativityZaara RyeenNo ratings yet
- Periodic Table and PeriodicityDocument11 pagesPeriodic Table and Periodicityakeemoluwadamilare623No ratings yet
- First Ionisation Energies (FIEsDocument6 pagesFirst Ionisation Energies (FIEsAathifa ThowfeekNo ratings yet
- Trends in atomic properties across the periodic tableDocument25 pagesTrends in atomic properties across the periodic tableHanna GalatiNo ratings yet
- Preodic Trends and Chemical ReactivityDocument8 pagesPreodic Trends and Chemical ReactivityBhavya SinghNo ratings yet
- Chem 111 Week Three Notes BDocument7 pagesChem 111 Week Three Notes BnyachiosirNo ratings yet
- 3 Periodic Trends For My WebpageDocument26 pages3 Periodic Trends For My WebpageApril Mergelle LapuzNo ratings yet
- Group 3: Periodic Relationship: Wendell Bandiola (THE PERIODIC TABLE)Document66 pagesGroup 3: Periodic Relationship: Wendell Bandiola (THE PERIODIC TABLE)Kristel Ann LaudeNo ratings yet
- Electron Configuration of IonsDocument8 pagesElectron Configuration of IonsGetnet BegashawNo ratings yet
- 3.2 Periodic TrendsDocument45 pages3.2 Periodic TrendsAli Mohamed ShiplNo ratings yet
- Atomic Radius and Ionization Energy TrendsDocument4 pagesAtomic Radius and Ionization Energy TrendsJDR JDRNo ratings yet
- Module 2 - Conduction and Breakdown in GasesDocument58 pagesModule 2 - Conduction and Breakdown in GasesFah RukhNo ratings yet
- A - Level Inorganic Chemistry 2-1Document171 pagesA - Level Inorganic Chemistry 2-1kitderoger_391648570No ratings yet
- Ionazation Energy GraphDocument15 pagesIonazation Energy GraphMaricel Cotanda EginaNo ratings yet
- Periodic Trends in Atomic PropertiesDocument41 pagesPeriodic Trends in Atomic Propertiessabhari_ramNo ratings yet
- Chem U1 M1 1.3 (Electronic Configuration)Document11 pagesChem U1 M1 1.3 (Electronic Configuration)Rachael FooteNo ratings yet
- Unit 1: Structure, Bonding and Main Group ChemistryDocument7 pagesUnit 1: Structure, Bonding and Main Group ChemistryJosh ColeNo ratings yet
- Classification of Elements PPT 11Document32 pagesClassification of Elements PPT 11Aditya SRIVASTAVANo ratings yet
- Periodic Table (4,5,6)Document37 pagesPeriodic Table (4,5,6)rehanfazal9669No ratings yet
- Periodic TrendsDocument19 pagesPeriodic TrendsgehgehkkNo ratings yet
- Atomic Radii: Atomic Structure: Periodic TrendsDocument5 pagesAtomic Radii: Atomic Structure: Periodic TrendsRajat SharmaNo ratings yet
- Atomic Theory 5Document5 pagesAtomic Theory 5Lateef TaiwoNo ratings yet
- Periodic Trends in Physical and Chemical PropertiesDocument68 pagesPeriodic Trends in Physical and Chemical PropertiesAnisha Syazwana Binti RoslyNo ratings yet
- Periodic Properties of The ElementsDocument57 pagesPeriodic Properties of The ElementstalktotiffanychengNo ratings yet
- Periodic ClassificationDocument36 pagesPeriodic ClassificationSHAIK YASMINNo ratings yet
- Ionization EnergyDocument8 pagesIonization EnergyHafiza Sikder AnishaNo ratings yet
- CHM 122 Notes 19 20Document21 pagesCHM 122 Notes 19 20Stephen VictorNo ratings yet
- HL Electron Configuration and The Periodic TableDocument12 pagesHL Electron Configuration and The Periodic TableMarilee HuntNo ratings yet
- 3 2 Physical PropertiesDocument4 pages3 2 Physical PropertiesNguyenHoangMinhDucNo ratings yet
- IonizationDocument23 pagesIonizationbalweg mackyNo ratings yet
- Zenith Tutorial - . - Making The Complex Simple : CALL/WHATSAPP EUREKA ON: 08149147222Document10 pagesZenith Tutorial - . - Making The Complex Simple : CALL/WHATSAPP EUREKA ON: 08149147222Oluwabamire OreoluwaNo ratings yet
- Cells Study Guide PDFDocument2 pagesCells Study Guide PDFhussein saripNo ratings yet
- Errors and Measurements in ChemistryDocument29 pagesErrors and Measurements in ChemistryOluwabamire Oreoluwa100% (1)
- Biology questions directed to 100 level students in MKOLDocument1 pageBiology questions directed to 100 level students in MKOLOluwabamire OreoluwaNo ratings yet
- Lec 27 PDFDocument7 pagesLec 27 PDFYalew MekonnenNo ratings yet
- Electrical Machines and Measurements Exam QuestionsDocument4 pagesElectrical Machines and Measurements Exam QuestionsTechno madNo ratings yet
- Liquid–Liquid Phase Separation in Polysulfone SystemsDocument11 pagesLiquid–Liquid Phase Separation in Polysulfone SystemsJamilly Ribeiro LopesNo ratings yet
- Basic On K-E ModelDocument7 pagesBasic On K-E ModelSayeem ZamanNo ratings yet
- Power Systems Protection Course: Al-Balqa Applied UniversityDocument31 pagesPower Systems Protection Course: Al-Balqa Applied UniversityDiego Del CastilloNo ratings yet
- Chapter3 PDFDocument57 pagesChapter3 PDFAmilvia Vega OrozcoNo ratings yet
- Kuliah 12 Aliran KompresibelDocument67 pagesKuliah 12 Aliran KompresibelherawanadifNo ratings yet
- Electrical Circuit Project Lab ReportDocument10 pagesElectrical Circuit Project Lab ReportrstiltneNo ratings yet
- ICG SSV - Simulation Phase 2 Link Budget Setup: Werner Enderle 05/06/2016Document13 pagesICG SSV - Simulation Phase 2 Link Budget Setup: Werner Enderle 05/06/2016Firas ZekiNo ratings yet
- 3rd Quarter Summative TestDocument4 pages3rd Quarter Summative TestSherine Kate SicariusNo ratings yet
- Characterisation of Earthing Systems Under High Frequency and Transient ConditionsDocument5 pagesCharacterisation of Earthing Systems Under High Frequency and Transient ConditionsStefanos DiamantisNo ratings yet
- r05211002 Electrical TechnologyDocument7 pagesr05211002 Electrical TechnologySRINIVASA RAO GANTANo ratings yet
- Structural Engineering FundamentalsDocument29 pagesStructural Engineering FundamentalsJoan ZullaNo ratings yet
- Chapter 4 - The Bernoulli Equation and Pressure VariationDocument20 pagesChapter 4 - The Bernoulli Equation and Pressure VariationErnesto LimNo ratings yet
- Pro/Ii Excel - Engine Unitid XXX: NcalDocument32 pagesPro/Ii Excel - Engine Unitid XXX: NcalYves-donald MakoumbouNo ratings yet
- Tabela Transformadas LaplaceDocument2 pagesTabela Transformadas LaplacePedro OliveiraNo ratings yet
- FM Unitwise Test AllDocument4 pagesFM Unitwise Test Allbodakevivek888No ratings yet
- CHM01a First TermDocument6 pagesCHM01a First TermMayce OngNo ratings yet
- English Test Results - CocubesDocument42 pagesEnglish Test Results - CocubesJavaharReddyNo ratings yet
- Newtonian Mechanics 5Document2 pagesNewtonian Mechanics 5Nachiketa SarkarNo ratings yet
- z198 201Document4 pagesz198 201mikiNo ratings yet
- The Heat Transfer Across A 5" Wall of Firebrick Is...Document3 pagesThe Heat Transfer Across A 5" Wall of Firebrick Is...MelindaNo ratings yet
- Physical Modeling of Sheet Piles Behavior To Improve Their Numerical Modeling and DesignDocument12 pagesPhysical Modeling of Sheet Piles Behavior To Improve Their Numerical Modeling and DesignAhmed RamadanNo ratings yet
- Physics Portion PIEAS 2013Document3 pagesPhysics Portion PIEAS 2013Shahzaib Arain100% (1)