Professional Documents
Culture Documents
Chemsheets AS 1033 Maxwell Boltzmann Curves
Uploaded by
charlesma123Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemsheets AS 1033 Maxwell Boltzmann Curves
Uploaded by
charlesma123Copyright:
Available Formats
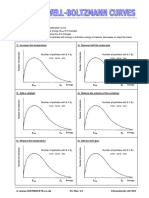
MAXWELL
MAXWELL-BOLTZMANN
BOLTZMANN CURVES
For each of the following
a) Sketch the new shape of the distribution curve
b) Indicate the new most probable energy (Emp) if it changes
c) Indicate the new activation energy (Ea) if it changes
d) Indicate whether the number of particles with energy ≥ activation energy increases, decreases or stays the same
1) Increase the temperature 2) Remove half the molecules (at constant temperature)
Number of molecules
Number of molecules
Number of particles with E ≥ Ea Number of particles with E ≥ Ea
more same less more same less
Emp Ea Energy Emp Ea Energy
3) Add a catalyst (at constant temperature) 4) Reduce the volume of the container (at constant temp.)
Number of molecules
Number of molecules
Number of particles with E ≥ Ea Number of particles with E ≥ Ea
more same less more same less
Emp Ea Energy Emp Ea Energy
5) Reduce the temperature 6) Add an inert gas (at constant temperature)
temperature
Number of molecules
Number of molecules
Number of particles with E ≥ Ea Number of particles with E ≥ Ea
more same less more same less
Emp Ea Energy Emp Ea Energy
© www.CHEMSHEETS.co.uk 11-December-2015 Chemsheets AS 1033
You might also like
- Chemsheets AS 1174 KC and Its Units ANS PDFDocument1 pageChemsheets AS 1174 KC and Its Units ANS PDFAlisha Shahid33% (3)
- As 1287 Group 7 PropertiesDocument2 pagesAs 1287 Group 7 Propertieskhadijah aliNo ratings yet
- Chemsheets AS 1089 (IR Problems 2) ANS Ameu74Document1 pageChemsheets AS 1089 (IR Problems 2) ANS Ameu74noramin89No ratings yet
- Chemsheets AS 1087 IR SpectrosDocument67 pagesChemsheets AS 1087 IR SpectrosjnfjngsdjNo ratings yet
- Unit 2 NotesDocument28 pagesUnit 2 NotesMuhammad ZaiNo ratings yet
- The Bromination of Acetone Lab ReportDocument4 pagesThe Bromination of Acetone Lab ReportSammy Njenga KhanNo ratings yet
- Datasheet de Motor de Paso M35SPDocument1 pageDatasheet de Motor de Paso M35SPMiguel VelezNo ratings yet
- Steeper MotorDocument1 pageSteeper MotorMiguel VelezNo ratings yet
- BCHN 213 Practical Exam 1 PreprationsDocument17 pagesBCHN 213 Practical Exam 1 Preprationskamohelo tsoeuNo ratings yet
- Chemsheets A2 1081 Acids and Bases Booklet ANSDocument27 pagesChemsheets A2 1081 Acids and Bases Booklet ANSjyumijacksonNo ratings yet
- Fuel CellsDocument75 pagesFuel CellsBaranidharan Gopi100% (1)
- Lesson Proper #5 Resonance and Formal ChargeDocument35 pagesLesson Proper #5 Resonance and Formal ChargeSamantha CamposNo ratings yet
- Complex IonsDocument4 pagesComplex Ionsmikey12345452863879No ratings yet
- GLP Practical 1a Calculation 2022 BCHN213 - MEMODocument3 pagesGLP Practical 1a Calculation 2022 BCHN213 - MEMOKAGISO BRIAN MOTSHUPHINo ratings yet
- Allen Major 1 QPDocument23 pagesAllen Major 1 QPelavarasanipadNo ratings yet
- Reactions of Aldehydes & Ketones: Oxidation & ReductionDocument4 pagesReactions of Aldehydes & Ketones: Oxidation & ReductionjnfjngsdjNo ratings yet
- Matriculation Chemistry (Aromatic Compound)Document87 pagesMatriculation Chemistry (Aromatic Compound)ridwanNo ratings yet
- 미즐러 (5판) 번역본Document798 pages미즐러 (5판) 번역본화학No ratings yet
- CHEG 320 - Electrode Kinetics - Extended NotesDocument13 pagesCHEG 320 - Electrode Kinetics - Extended NotesAzzkikrasdfNo ratings yet
- BCH2601 - 23 - Assignment 2Document8 pagesBCH2601 - 23 - Assignment 2freislichjpNo ratings yet
- Gaseous State For IIT AdancedDocument37 pagesGaseous State For IIT AdancedSube DevindaNo ratings yet
- Chemsheets A2 1029 (Catalysis)Document17 pagesChemsheets A2 1029 (Catalysis)Jon HadleyNo ratings yet
- Chemsheets AS 1077 Petroleum and AlkanesDocument4 pagesChemsheets AS 1077 Petroleum and Alkanescharlesma123No ratings yet
- IB Chemistry - Exam Guide With Key Points - Sample PagesDocument14 pagesIB Chemistry - Exam Guide With Key Points - Sample PagesjoyceNo ratings yet
- A2 CHM Sol 05 Acid and Base WSDocument28 pagesA2 CHM Sol 05 Acid and Base WSnsNo ratings yet
- HW #8 - SolutionDocument2 pagesHW #8 - SolutionMatty JakeNo ratings yet
- 05 Acid Base and Buffer WS 2021Document37 pages05 Acid Base and Buffer WS 2021VayaNo ratings yet
- Version A: Name: - Section: - TA Name: - Chemistry 237 Exam 1Document10 pagesVersion A: Name: - Section: - TA Name: - Chemistry 237 Exam 1Eugene SimNo ratings yet
- Datasheet Motor VideoDocument1 pageDatasheet Motor VideoDocente Fede TecnologicoNo ratings yet
- Collision TheoryDocument10 pagesCollision TheoryAnonymous pgjIAZoNo ratings yet
- Alkenes Alkynes Oxidation PDFDocument32 pagesAlkenes Alkynes Oxidation PDFRamuNo ratings yet
- Structure 1.1, 1.2, 1.3 PracticeDocument6 pagesStructure 1.1, 1.2, 1.3 PracticeEthan ElliotNo ratings yet
- 40 Conductivity Meter LMCM 20 PDFDocument1 page40 Conductivity Meter LMCM 20 PDFrajap02No ratings yet
- Unit 3B A Level Chemistry Revision NotesDocument13 pagesUnit 3B A Level Chemistry Revision NotesHakim AbbasNo ratings yet
- 1018 Combo Batch PDFDocument4 pages1018 Combo Batch PDFAnjali GuptaNo ratings yet
- Body Fluids and Circulation by Dr. Sunita SaxenaDocument71 pagesBody Fluids and Circulation by Dr. Sunita SaxenaDivya AgarawalNo ratings yet
- NMR Booklet QuestionsDocument21 pagesNMR Booklet QuestionsSumaira AliNo ratings yet
- Chemistry Unit 2 Part 3 ReallyacademicsDocument78 pagesChemistry Unit 2 Part 3 ReallyacademicsWill AndyNo ratings yet
- A Level Benzene NotesDocument1 pageA Level Benzene NotesSonnyMaanNo ratings yet
- Assignment 8 - Solutions Chem1000ADocument4 pagesAssignment 8 - Solutions Chem1000AXdyne67% (3)
- OCW Exam 1Document10 pagesOCW Exam 1iliketospam123No ratings yet
- NCERT Notes For Class 12 Chemistry Chapter 2: Solutions: Solute and SolventDocument11 pagesNCERT Notes For Class 12 Chemistry Chapter 2: Solutions: Solute and Solventshradha bittuNo ratings yet
- Lecture 8F M.SC - SEM IVDocument6 pagesLecture 8F M.SC - SEM IVVishalNo ratings yet
- IC 211 Lab Manual 2018-2019Document102 pagesIC 211 Lab Manual 2018-2019AdityaSoniNo ratings yet
- Physics A2 FormulasDocument11 pagesPhysics A2 FormulasDishitaNo ratings yet
- הרצאה 6Document8 pagesהרצאה 6api-26922789No ratings yet
- MIT5 111F14 ProbReviewDocument3 pagesMIT5 111F14 ProbReviewMD Abu RaselNo ratings yet
- Atomic Structure (AP MC)Document4 pagesAtomic Structure (AP MC)Nyxas IoannisNo ratings yet
- Bi-Bi RXNDocument12 pagesBi-Bi RXNbelabelawNo ratings yet
- (ED) .DC Amp Design With FETs Zero-TCDocument5 pages(ED) .DC Amp Design With FETs Zero-TCf826401No ratings yet
- Paper 1 Section B Question-Answer Book BDocument20 pagesPaper 1 Section B Question-Answer Book BKathy WongNo ratings yet
- Past Paper Micro World I II 2019 20 PDFDocument19 pagesPast Paper Micro World I II 2019 20 PDF779720 cNo ratings yet
- CH 07Document96 pagesCH 07Jacquot AbendrothNo ratings yet
- 9bn0 01 Que 20230608Document32 pages9bn0 01 Que 20230608Ahmed AbdelrhemNo ratings yet
- Chemsheets AS 095 (Maxwell-Boltmann Curves)Document1 pageChemsheets AS 095 (Maxwell-Boltmann Curves)Srikaushik TumulaNo ratings yet
- Reaction Rate Notes CompletDocument57 pagesReaction Rate Notes CompletTasha AusmanNo ratings yet
- Reaction Rates and Temperature Arrhenius Theory: CHEM 102 T. HughbanksDocument12 pagesReaction Rates and Temperature Arrhenius Theory: CHEM 102 T. HughbanksIAS IndiaNo ratings yet
- Revision Notes - Unit 2 AQA Chemistry A-LevelDocument16 pagesRevision Notes - Unit 2 AQA Chemistry A-LevelWajid AliNo ratings yet
- Topic 5 - Energetics - ThermochemistryDocument7 pagesTopic 5 - Energetics - Thermochemistrycharlesma123No ratings yet
- Updated-Pearson Edexcel Global Online Training-FreeDocument62 pagesUpdated-Pearson Edexcel Global Online Training-Freecharlesma123No ratings yet
- 学教翻转的课堂⻛风景 Perspective of Flipped Learning: - 融侨赛德伯学校总校⻓长 林林莘 Rong Qiao Sedbergh School, Kelly LinDocument24 pages学教翻转的课堂⻛风景 Perspective of Flipped Learning: - 融侨赛德伯学校总校⻓长 林林莘 Rong Qiao Sedbergh School, Kelly Lincharlesma123No ratings yet
- Cambridge Professional Development Qualifications: 剑桥国际教师专业发展认证课程Document15 pagesCambridge Professional Development Qualifications: 剑桥国际教师专业发展认证课程charlesma123No ratings yet
- David RAISE分论坛7 演讲3Document21 pagesDavid RAISE分论坛7 演讲3charlesma123No ratings yet
- 分论坛3 ChistopKKKKMoses DKKDocument12 pages分论坛3 ChistopKKKKMoses DKKcharlesma123No ratings yet
- 拥抱不不确定性:如何做好疫情下的外教⽀支持 Embracing Uncertainty : How to best support international faculty in the pandemicDocument19 pages拥抱不不确定性:如何做好疫情下的外教⽀支持 Embracing Uncertainty : How to best support international faculty in the pandemiccharlesma123No ratings yet
- 9701 - s17 - QP - 42 CharlesDocument30 pages9701 - s17 - QP - 42 Charlescharlesma123No ratings yet
- Pearson Edexcel Cloud ClassDocument3 pagesPearson Edexcel Cloud Classcharlesma123No ratings yet
- 12 - An Introduction To The Chemistry of Transition ElementsDocument46 pages12 - An Introduction To The Chemistry of Transition Elementscharlesma123No ratings yet
- 9701 TP3 Aldehydes v2.0Document30 pages9701 TP3 Aldehydes v2.0charlesma123No ratings yet
- 2010 Paper, Question 1Document2 pages2010 Paper, Question 1charlesma123No ratings yet
- Pearson Edexcel EPQ Online Training by DR John TaylorDocument1 pagePearson Edexcel EPQ Online Training by DR John Taylorcharlesma123No ratings yet
- Cambridge International AS & A Level: CHEMISTRY 9701/34Document16 pagesCambridge International AS & A Level: CHEMISTRY 9701/34charlesma123No ratings yet
- 9701 TP4 Alkenes v2.0Document38 pages9701 TP4 Alkenes v2.0charlesma123No ratings yet
- Little Difficulty: Answer(s) CorrectDocument1 pageLittle Difficulty: Answer(s) Correctcharlesma123No ratings yet
- Ig Chem ALL EQ P6 17w To 16m Labelling eDocument14 pagesIg Chem ALL EQ P6 17w To 16m Labelling echarlesma123No ratings yet
- Kiln Performance & Efficiency Formulas (Updated and Completed) - InFINITY For CEMENT EQUIPMENTDocument3 pagesKiln Performance & Efficiency Formulas (Updated and Completed) - InFINITY For CEMENT EQUIPMENTBùi Hắc HảiNo ratings yet
- Stulz Cyberair 3pro DX Asr Brochure 1805 enDocument12 pagesStulz Cyberair 3pro DX Asr Brochure 1805 enMudassar Idris RautNo ratings yet
- Mold Temperature ControlDocument6 pagesMold Temperature ControlmalarmanicNo ratings yet
- AP Chemistry Multiple-Choice Review Tips NotesDocument4 pagesAP Chemistry Multiple-Choice Review Tips Noteserwin golovashkinNo ratings yet
- Haba Pendam PelakuranDocument3 pagesHaba Pendam PelakuranSOON MEY CHE KPM-Guru0% (1)
- IB Chemistry SL Topic 5 Questions 1Document11 pagesIB Chemistry SL Topic 5 Questions 1Vibha RaviNo ratings yet
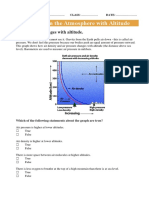
- Altitude and Atmospheric Changes WorksheetDocument3 pagesAltitude and Atmospheric Changes WorksheetJohn Osborne0% (1)
- 100 Years Trane HistoryDocument8 pages100 Years Trane HistoryjamosrdonNo ratings yet
- MATE 152-24 Lec 2Document25 pagesMATE 152-24 Lec 2andrew.dungoNo ratings yet
- AssimentDocument3 pagesAssimentSantosh SharmaNo ratings yet
- Information/Data Required For Wax Modelling: A) B) C) D) E)Document6 pagesInformation/Data Required For Wax Modelling: A) B) C) D) E)AYAUWU LOVEDAY100% (1)
- Cpe Notes - Unit IIDocument4 pagesCpe Notes - Unit IIdhananivethaNo ratings yet
- Principles of Heat Transfer Solutions ManualDocument783 pagesPrinciples of Heat Transfer Solutions ManualaditNo ratings yet
- Extended Surfaces / FinsDocument35 pagesExtended Surfaces / FinscaptainhassNo ratings yet
- De Paula 2020Document11 pagesDe Paula 2020Sangameshwaran SadhasivamNo ratings yet
- LabreportphysicdDocument4 pagesLabreportphysicdapi-263436863No ratings yet
- Chapter 5.2 Form 1 KSSSM ScienceDocument4 pagesChapter 5.2 Form 1 KSSSM ScienceCt Sophie Phea100% (1)
- Effect of Air Velocity On Drying Process (Group 1&2)Document7 pagesEffect of Air Velocity On Drying Process (Group 1&2)Sofea IzyanNo ratings yet
- International Journal of Heat and Mass Transfer: Vedran Medica-Viola, Branimir Pavkovic, Vedran MrzljakDocument12 pagesInternational Journal of Heat and Mass Transfer: Vedran Medica-Viola, Branimir Pavkovic, Vedran MrzljakVamsi DeepakNo ratings yet
- Shell and Tube Heat ExchangerDocument112 pagesShell and Tube Heat Exchangerramesh pokhrel100% (3)
- Sala FormatDocument82 pagesSala FormatLemuel ReñaNo ratings yet
- AzeotrophsDocument5 pagesAzeotrophsMohit PassiNo ratings yet
- Celsius Brochure 8.5x11 PDFDocument8 pagesCelsius Brochure 8.5x11 PDFFLIRIDIR1678No ratings yet
- Types of Plug Flow ReactorsDocument7 pagesTypes of Plug Flow ReactorsDhyrana Shaila100% (1)
- Chemical Engineering Thermodynamics Project-I: TopicDocument11 pagesChemical Engineering Thermodynamics Project-I: TopicRohit GuptaNo ratings yet
- ME 205 ThermodynamicsDocument3 pagesME 205 Thermodynamicsnandan144No ratings yet
- ER CH3-Student 2022Document45 pagesER CH3-Student 2022aboem16No ratings yet
- Text 9Document9 pagesText 9Debela mendaraNo ratings yet
- Ug Ece 2020Document185 pagesUg Ece 2020Dr. Anoop Jacob ThomasNo ratings yet