Professional Documents
Culture Documents
As 1287 Group 7 Properties
Uploaded by
khadijah aliCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
As 1287 Group 7 Properties
Uploaded by
khadijah aliCopyright:
Available Formats
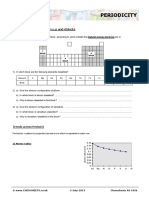
GROUP 7 - PROPERTIES
fluorine (F2) chlorine (Cl2) bromine (Br2) Iodine (I2)
appearance yellow gas green gas brown liquid grey crystalline solid
comments • very easily forms orange • very easily forms purple vapour
vapour
• often used as a (sort of)
• often used as a solution in solution in water (brown “iodine
water (yellow-orange bromine solution”)
water)
Atomic radius
160
140
Atomic radius (pm)
120
100
80
60
40
20
0
fluorine chlorine bromine iodine
Electronegativity
4
3.5
3
Electronegativity
2.5
2
1.5
1
0.5
0
fluorine chlorine bromine iodine astatine
Melting & boiling points
400
300
200
Temperature (°C)
100 melting point (°C)
0 boiling point (°C)
fluorine chlorine bromine iodine astatine
-100
-200
-300
© www.CHEMSHEETS.co.uk 03-Feb-2021 Chemsheets AS 1287
1st ionisation energy
1800
1600
Ionisation energy (kJ/mol)
1400
1200
1000
800
600
400
200
0
F Cl Br I At
Reactions of chlorine with water and NaOH(aq)
Equation Comments
Water
NaOH(aq)
(cold, dilute)
© www.CHEMSHEETS.co.uk 03-Feb-2021 Chemsheets AS 1287
You might also like
- Challenges in Management of Pollution Control in Electroplating Industries in Achieving Zero Liquid Discharge For Sustainable DevelopmentDocument27 pagesChallenges in Management of Pollution Control in Electroplating Industries in Achieving Zero Liquid Discharge For Sustainable Developmentprashant_cool_4_uNo ratings yet
- Introduction to Cooling Water Treatment Parameters and IndicesDocument40 pagesIntroduction to Cooling Water Treatment Parameters and IndicessomaniNo ratings yet
- 23-The Solubility of Kraft Recovery Boiler Precipitator Ash-Daniel SaturninoDocument37 pages23-The Solubility of Kraft Recovery Boiler Precipitator Ash-Daniel Saturninozhiguang huangNo ratings yet
- 2.3. Group 7 (17) The HalogensDocument2 pages2.3. Group 7 (17) The Halogenshaseeb3382786No ratings yet
- Student Projects For B.Sc. Chemistry: Dr. R. Rajeev VSSC, ThiruvananthapuramDocument28 pagesStudent Projects For B.Sc. Chemistry: Dr. R. Rajeev VSSC, ThiruvananthapuramNishantNo ratings yet
- Drinking Water Booklet - 05-14-2007Document152 pagesDrinking Water Booklet - 05-14-2007Minos_CoNo ratings yet
- 20l237 Evs Exp5Document4 pages20l237 Evs Exp5Prajit .TNo ratings yet
- Stasis Device CalculatorDocument7 pagesStasis Device CalculatorSamuel RubinNo ratings yet
- Elements of Group ViiDocument20 pagesElements of Group ViiNelima Stella mercyNo ratings yet
- Corrosion: USNA Chemistry DepartmentDocument18 pagesCorrosion: USNA Chemistry DepartmentFaraj HaiderNo ratings yet
- Corrosion FundamentalsDocument113 pagesCorrosion FundamentalsahmadhatakeNo ratings yet
- Analytical Portfolio For Mining ApplicationsDocument35 pagesAnalytical Portfolio For Mining ApplicationsLud PasNo ratings yet
- ChemistryDocument3 pagesChemistryRosanna SessionsNo ratings yet
- SaltworksDocument2 pagesSaltworksChapNo ratings yet
- Presentation ICDocument32 pagesPresentation ICgitaismala90No ratings yet
- ElectrolysisDocument3 pagesElectrolysisMohit RawatNo ratings yet
- NON-FERROUS Extractive FinalDocument40 pagesNON-FERROUS Extractive FinalJaidevNo ratings yet
- Electro-Membrane Salt Splitting Process To Produce Lithium HydroxideDocument12 pagesElectro-Membrane Salt Splitting Process To Produce Lithium HydroxideazturanNo ratings yet
- BOILERS WATER TREATMENT CHEMISTRYDocument38 pagesBOILERS WATER TREATMENT CHEMISTRYMahmoud MahmoudmNo ratings yet
- Screening Damage Mechanism - 581 & 571Document28 pagesScreening Damage Mechanism - 581 & 571Febri Ramdani NugrahaNo ratings yet
- Important Diagrams 2 - Senarai Eksperimen KimiaDocument7 pagesImportant Diagrams 2 - Senarai Eksperimen Kimiadasima83No ratings yet
- Recycling of Metals From Spent Catalyst PDFDocument6 pagesRecycling of Metals From Spent Catalyst PDFElsherif AlyNo ratings yet
- Lecture 15.4 - Electrochem PhenomDocument5 pagesLecture 15.4 - Electrochem PhenomLiam DoranNo ratings yet
- Boilers Water Treatment: Chemist / Mustafa Ateia MustafaDocument38 pagesBoilers Water Treatment: Chemist / Mustafa Ateia MustafaMakhdoom Ibad HashmiNo ratings yet
- Jotun CourseDocument76 pagesJotun CourseElhusseiny Fouda100% (1)
- Chilled Water PresentationDocument20 pagesChilled Water PresentationPANDIARAJ KARUPPATHEVARNo ratings yet
- Adobe Scan 02-Jul-2021Document3 pagesAdobe Scan 02-Jul-2021Uppada LaxmiNo ratings yet
- Ammonium Sulphate: Commercial Form, Storage and TransportationDocument4 pagesAmmonium Sulphate: Commercial Form, Storage and TransportationPar PatelNo ratings yet
- Ammonium Sulfate by Direct Route PDFDocument4 pagesAmmonium Sulfate by Direct Route PDFsandipkumardshahNo ratings yet
- Chlor Alkali TechnologyDocument14 pagesChlor Alkali TechnologyTinTin100% (1)
- Revision Sheet - CSECDocument14 pagesRevision Sheet - CSECTia-marie Mc AlisterNo ratings yet
- Water Technology Requirements and ParametersDocument12 pagesWater Technology Requirements and ParametersKARTHI KRISHNANo ratings yet
- Hydrothermal Process PPT 4Document22 pagesHydrothermal Process PPT 4Awais AhmadNo ratings yet
- Gerspacher MichelDocument19 pagesGerspacher MichelPratik BhagatNo ratings yet
- Inorganic Chemistry: Group 13 AluminiumDocument38 pagesInorganic Chemistry: Group 13 AluminiumLooi Chui Yean0% (1)
- MetalDocument57 pagesMetalPrashant PuriNo ratings yet
- Specimen QP - GCSE Higher Chemistry 1HDocument34 pagesSpecimen QP - GCSE Higher Chemistry 1Hrekterer87youNo ratings yet
- ElectroDocument48 pagesElectroMang friesNo ratings yet
- HalogenDocument37 pagesHalogenPutri Dierla Dela100% (1)
- NAME: Kaixin Jervey A. Ventura SECTION: 7-Sapphire: WORKSHEET in Science 7Document7 pagesNAME: Kaixin Jervey A. Ventura SECTION: 7-Sapphire: WORKSHEET in Science 7Meynard Garcia CastroNo ratings yet
- Electrowinning Precious Metals From Cyanide Solutions Using Emew TechnologyDocument24 pagesElectrowinning Precious Metals From Cyanide Solutions Using Emew TechnologyJOSE MACASSINo ratings yet
- Raw Water Treatment Filtration ProcessDocument38 pagesRaw Water Treatment Filtration ProcessRuang RenungNo ratings yet
- Characteristics of Boiler Feedwater - An Overview of Key Water Quality ParametersDocument1 pageCharacteristics of Boiler Feedwater - An Overview of Key Water Quality ParametersjagjitNo ratings yet
- Group 7 - Physical Properties: Www. .CO - UKDocument10 pagesGroup 7 - Physical Properties: Www. .CO - UKcharlesma123No ratings yet
- Davidmccormack Shell - Subjectmatterexpert - Failure Diagnosis & TroubleshootingDocument39 pagesDavidmccormack Shell - Subjectmatterexpert - Failure Diagnosis & TroubleshootingAkmaral100% (1)
- Aluminum-Beryllium Alloys For Aerospace Applications: Materion Corporation Materion Beryllium & Composites 14710Document7 pagesAluminum-Beryllium Alloys For Aerospace Applications: Materion Corporation Materion Beryllium & Composites 14710roshniNo ratings yet
- Distillery Waste Water Treatment: Praveen Kumar ToniDocument26 pagesDistillery Waste Water Treatment: Praveen Kumar ToniMehdi Hassan MiluNo ratings yet
- Simchem 388Document1 pageSimchem 388erkinongulNo ratings yet
- Corrosion AtmosDocument18 pagesCorrosion AtmosFajri Febriano MuktiNo ratings yet
- Plant Tender EZ OffDocument16 pagesPlant Tender EZ OffGowri GaneshNo ratings yet
- Ferric Chloride ProperitiesDocument4 pagesFerric Chloride ProperitiesShaaban NoamanNo ratings yet
- Water Conditioning Process ExplainedDocument27 pagesWater Conditioning Process ExplainedKamran RanaNo ratings yet
- CCE14 - Testing Salts For Anions and Student Handout PDFDocument3 pagesCCE14 - Testing Salts For Anions and Student Handout PDFvNo ratings yet
- Temperature ThresholdDocument4 pagesTemperature Thresholdkenny_1983_lfyNo ratings yet
- Metal Extraction Process OverviewDocument5 pagesMetal Extraction Process OverviewFARAHFATINZNo ratings yet
- MDMW Cobalt01Document3 pagesMDMW Cobalt01miningnovaNo ratings yet
- Flowchart 23Document17 pagesFlowchart 23gboopathy123No ratings yet
- Lecture No. 30: Subject: Concrete Durability Problems - Objectives of LectureDocument17 pagesLecture No. 30: Subject: Concrete Durability Problems - Objectives of LectureRaunak TimilsinaNo ratings yet
- Chemistry Factsheet (OL, IGCSE, MYP) FinalDocument19 pagesChemistry Factsheet (OL, IGCSE, MYP) Finalcreate your own gaming worldNo ratings yet
- Spectroscopy and Photochemistry of Uranyl Compounds: International Series of Monographs on Nuclear EnergyFrom EverandSpectroscopy and Photochemistry of Uranyl Compounds: International Series of Monographs on Nuclear EnergyNo ratings yet
- CL 3 Volumes of PrismsDocument1 pageCL 3 Volumes of PrismsMartin MalinovNo ratings yet
- Ch.10 Forces and MotionDocument1 pageCh.10 Forces and Motionkhadijah aliNo ratings yet
- 3.8 Revision Guide Aldehydes and Ketones AqaDocument3 pages3.8 Revision Guide Aldehydes and Ketones Aqakhadijah aliNo ratings yet
- AS PeriodicityDocument2 pagesAS Periodicitykhadijah aliNo ratings yet
- AS 1059 Group 2 WaterDocument1 pageAS 1059 Group 2 Waterkhadijah aliNo ratings yet
- Third Term Chemistry SS1Document75 pagesThird Term Chemistry SS1Sunday Ozovehe100% (1)
- Study Finds Environmentally-Friendly Coating Improves Corrosion Resistance on Electrical SteelDocument8 pagesStudy Finds Environmentally-Friendly Coating Improves Corrosion Resistance on Electrical SteelmaNo ratings yet
- THE COMPLETE Chemistry Notes for Railway ExamsDocument39 pagesTHE COMPLETE Chemistry Notes for Railway ExamsPravinNo ratings yet
- Centella Asiatica Menggunakan Fosfatidilkolin: Formulasi Liposom Ekstrak Terpurifikasi Dan KolesterolDocument5 pagesCentella Asiatica Menggunakan Fosfatidilkolin: Formulasi Liposom Ekstrak Terpurifikasi Dan Kolesterolsiska putri utamaNo ratings yet
- Wound DressingDocument42 pagesWound DressingAbdulazeez Abdulmalik100% (1)
- Master Catalog PetroDocument76 pagesMaster Catalog PetroAlexSNo ratings yet
- Earth and Life ScienceDocument30 pagesEarth and Life ScienceChristian VitalesNo ratings yet
- 5C - Stoichiometry 3Document38 pages5C - Stoichiometry 3Vimanan A/L S. VelangganiNo ratings yet
- IS 1891-1978 Part-4 R-2005Document3 pagesIS 1891-1978 Part-4 R-2005Deepjyoti DasNo ratings yet
- Acenocoumarol Guidance With Conversion InfoDocument2 pagesAcenocoumarol Guidance With Conversion InfoMihu DragostinNo ratings yet
- Aloe Vera in Preservation of StrawberryDocument13 pagesAloe Vera in Preservation of StrawberryPhan Nguyễn TrangNo ratings yet
- PRK1016_STOICHIOMETRYDocument3 pagesPRK1016_STOICHIOMETRYTiong Chiong KianNo ratings yet
- ACM Gas Compressed 1Document6 pagesACM Gas Compressed 1Vinodhkanna GandhiNo ratings yet
- 30 HMA DatasheetDocument2 pages30 HMA DatasheetCricri CriNo ratings yet
- The Effect of Iron in Al-Si Casting AlloysDocument11 pagesThe Effect of Iron in Al-Si Casting AlloysJothi ManiNo ratings yet
- Quality Improvement of Corn Husk As Raw Material For Textile ProductsDocument5 pagesQuality Improvement of Corn Husk As Raw Material For Textile ProductsSophie BaromanNo ratings yet
- Subtopic 6.1: Polymers: MaterialsDocument32 pagesSubtopic 6.1: Polymers: MaterialschiggsNo ratings yet
- Worksheet-Nernst Equation PDFDocument4 pagesWorksheet-Nernst Equation PDFLedd SleddNo ratings yet
- Biology I: Multiple Choice Questions 20 Items Name: - SchoolDocument3 pagesBiology I: Multiple Choice Questions 20 Items Name: - SchoolEJ RaveloNo ratings yet
- Chemistry: Long Exam 1Document4 pagesChemistry: Long Exam 1Barbara BananaNo ratings yet
- Jawapan Soalan Ramalan Sains Kertas 2Document8 pagesJawapan Soalan Ramalan Sains Kertas 2syahidatul nurhanani100% (1)
- Characterisation of Fluorescence Background in Dye TracingDocument7 pagesCharacterisation of Fluorescence Background in Dye TracingALCIRA VALERIA CÉSPEDES VARGASNo ratings yet
- Toe To Heal Air Injection - THAIDocument5 pagesToe To Heal Air Injection - THAIAnthon100% (2)
- NCHE111 Study Guide 2024Document96 pagesNCHE111 Study Guide 2024sibulelemathandabuzo1No ratings yet
- PB Alumec EnglishDocument12 pagesPB Alumec EnglishByron RodriguezNo ratings yet
- Chemistry Booklet Science and Fun Part 1Document102 pagesChemistry Booklet Science and Fun Part 1ext.xd6948No ratings yet
- Restoring Polluted Lakes Using Structural and Non-Structural ApproachesDocument145 pagesRestoring Polluted Lakes Using Structural and Non-Structural ApproachesSugumar BalasubramaniamNo ratings yet
- Propagation MediaDocument38 pagesPropagation MediaHumayne SutherlandNo ratings yet
- Thermochemistry of Fuel & StoichiometricDocument32 pagesThermochemistry of Fuel & Stoichiometricasmaaasmaaa asmaaNo ratings yet
- Public Use of LifeAfter Profits - RedditDocument94 pagesPublic Use of LifeAfter Profits - RedditChristian DevanoNo ratings yet