100% found this document useful (3 votes)
4K views26 pagesGENERAL PHYSICS 1 Quarter 2 Activity Sheet
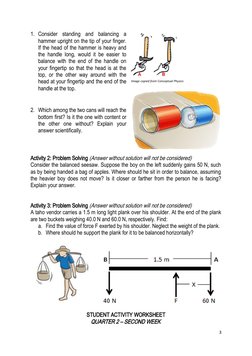
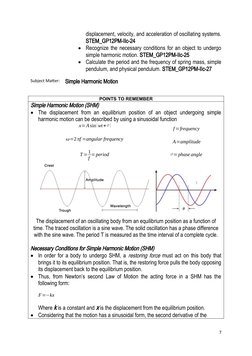
This student activity worksheet covers rotational motion and Newton's law of gravitation for a 12th grade physics class. The first section provides definitions and formulas for moment of inertia and calculating torque. Sample problems ask about balancing objects and finding forces. The second section summarizes Newton's law of gravitation, gravitational fields, and orbital mechanics. It defines key terms like gravitational potential energy and Kepler's laws of planetary motion. Practice problems apply these concepts to calculate gravitational acceleration and escape velocity.
Uploaded by
Severus S PotterCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
100% found this document useful (3 votes)
4K views26 pagesGENERAL PHYSICS 1 Quarter 2 Activity Sheet
This student activity worksheet covers rotational motion and Newton's law of gravitation for a 12th grade physics class. The first section provides definitions and formulas for moment of inertia and calculating torque. Sample problems ask about balancing objects and finding forces. The second section summarizes Newton's law of gravitation, gravitational fields, and orbital mechanics. It defines key terms like gravitational potential energy and Kepler's laws of planetary motion. Practice problems apply these concepts to calculate gravitational acceleration and escape velocity.
Uploaded by
Severus S PotterCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd