Professional Documents
Culture Documents
Formulazio Ez-Organikoa (Oxidoak)
Uploaded by
Hirune A0 ratings0% found this document useful (0 votes)
17 views2 pagesOriginal Title
Formulazio ez-organikoa (oxidoak)
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
17 views2 pagesFormulazio Ez-Organikoa (Oxidoak)
Uploaded by
Hirune ACopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
FORMULAZIO EZ-ORGANIKOA
a. OXIGENOAREN KONPOSATU BITARRAK:
1. Formulatu hurrengo konposatuak
a. Berun oxidoa:
b. Kromo monoxidoa:
c. Nitrógeno pentaoxidoa:
d. Platino (IV) oxidoa:
e. Estainu (II) oxidoa:
f. Sufre (IV) oxidoa:
g. Dimerkurio oxidoa:
h. Dizilar oxidoa:
i. Karbono (IV) oxidoa:
j. Boro (III) oxidoa:
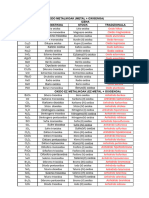
2. Izendatu hurrengo konposatuak
Izena Izena
Formula
(Aurrizkiak erabiliz) (Oxidazio zenbakiak erabiliz)
Co2O3
As2O3
MnO
ZnO
TeO2
Ni2O3
SnO2
P2O5
SiO2
CO
3. Izendatu edo formulatu
CuO.................................................................................................................
Cu2O................................................................................................................
FeO.................................................................................................................
Fe2O3...............................................................................................................
CaO...............................................................................................................
Merkurio (III) oxidoa......................................................................................
Litio oxidoa.....................................................................................................
Magnesio monoxidoa..................................................................................
Bario oxidoa ...................................................................................................
CO2..................................................................................................................
I2O5..................................................................................................................
SO2..................................................................................................................
Cl2O7................................................................................................................
SO3..................................................................................................................
Dikloro trioxidoa.............................................................................................
Bromo (III) oxidoa…………………………………………………………………………………......
K2O………………………………………………………………………………………………………….
SrO………………………………………………………………………………………………………….
Al2O3………………………………………………………………………………………………………….
CO2………………………………………………………………………………………………………….
SnO………………………………………………………………………………………………………….
PbO2………………………………………………………………………………………………………….
N2O………………………………………………………………………………………………………….
SO3………………………………………………………………………………………………………….
O5Br2…………………………………………………………………………………………………………
CoO………………………………………………………………………………………………………….
Fe2O3…………………………………………………………………………………………………………
Ag2O………………………………………………………………………………………………………….
CO………………………………………………………………………………………………………….
TeO2………………………………………………………………………………………………………….
You might also like
- Formulazio Ariketak (1) ZUZENKETAKDocument5 pagesFormulazio Ariketak (1) ZUZENKETAKIker Moreno RojasNo ratings yet
- Formulazio AriketakDocument3 pagesFormulazio AriketakAlfonso HernangilNo ratings yet
- Formulazio KimikaDocument3 pagesFormulazio KimikaAnaNo ratings yet
- Formulazio Inorganikoa 2017-18Document16 pagesFormulazio Inorganikoa 2017-18api-359727056No ratings yet
- 2 Oxigenoaren Konbinazio Bitarrak EmaitzakDocument1 page2 Oxigenoaren Konbinazio Bitarrak EmaitzakcristinaNo ratings yet
- ARIKETAK 1 - EmaitzakDocument4 pagesARIKETAK 1 - Emaitzakanderguti2008No ratings yet
- 1 - OXIDOAK Eta Anhidridoak IIDocument2 pages1 - OXIDOAK Eta Anhidridoak IIIñaki Zarate GonzalezNo ratings yet
- 04 Peroxidoak emDocument1 page04 Peroxidoak emjosu mañarikuaNo ratings yet
- Formulazioa Birpasoko Ariketak. ZuzenketaDocument2 pagesFormulazioa Birpasoko Ariketak. ZuzenketaTelmo Martinez AbaigarNo ratings yet
- 4 Gaia Formulazioa 4Document11 pages4 Gaia Formulazioa 4Ivan García BerasateguiNo ratings yet
- Emaitzak Formulazio Ez OrganikoaDocument6 pagesEmaitzak Formulazio Ez OrganikoaRiad MaalmineNo ratings yet
- 17-18 NOMENKLATURA DBH 4 (Ebatzita)Document11 pages17-18 NOMENKLATURA DBH 4 (Ebatzita)ESTHERNo ratings yet
- Teoria Formulazio Ez-OrganikoaDocument20 pagesTeoria Formulazio Ez-Organikoa01- Joseba Zabala Martin100% (1)
- Oxidoak Eta HidruroakDocument10 pagesOxidoak Eta HidruroakidoialazaroNo ratings yet
- AZIDO OXOAZIDOAK - SalbuespenakDocument1 pageAZIDO OXOAZIDOAK - SalbuespenakfqirakasNo ratings yet
- Hidruro MetalikoakDocument2 pagesHidruro MetalikoakAdrián Lozón, SantiagoNo ratings yet
- Formulazio FitxakDocument5 pagesFormulazio FitxakinfogelairekiaNo ratings yet
- Formulazio Ezorganikoa PDFDocument14 pagesFormulazio Ezorganikoa PDFUXUE PORRASNo ratings yet
- 2. Fitxa. Hidruro metalikoak, Haluroak eta Hidruro hegazkorrak fitxategiaren kopiaDocument3 pages2. Fitxa. Hidruro metalikoak, Haluroak eta Hidruro hegazkorrak fitxategiaren kopiagomezmerinounaiNo ratings yet
- Birpasatzeko Ariketak (1eba) 2021 22Document2 pagesBirpasatzeko Ariketak (1eba) 2021 22Laura HernándezNo ratings yet
- Formulazioa Eta Nomenklatura AzterketaDocument2 pagesFormulazioa Eta Nomenklatura AzterketaEJAIRAM5No ratings yet
- Formulazioa TeoriaDocument13 pagesFormulazioa TeoriaXabiNo ratings yet
- Formulaketa Inorganikoa El Carmelo Ikastetxea: Hidroxidoak Metala + Oh TaldeaDocument12 pagesFormulaketa Inorganikoa El Carmelo Ikastetxea: Hidroxidoak Metala + Oh TaldeaEztizen Bellón BarandikaNo ratings yet
- Formu Bitarrak Froga 1Document2 pagesFormu Bitarrak Froga 1Jon Etxebarria ArkarazoNo ratings yet
- 1 - Oxidoak Eta Anhidridoak IDocument14 pages1 - Oxidoak Eta Anhidridoak IAgurtzane IturbeNo ratings yet
- Formulazio Ez OrganikoaDocument8 pagesFormulazio Ez OrganikoaJara EspumosaNo ratings yet
- Formulazio AriketakDocument5 pagesFormulazio AriketakPeio IraolagoitiaNo ratings yet
- Kromo (IV) OxidoaDocument10 pagesKromo (IV) OxidoaMALEN ILLARREGINo ratings yet
- 2005 ArikformulazioezorganikoaDocument13 pages2005 ArikformulazioezorganikoaAnaNo ratings yet
- 2005 errepasoFORMULAZEZORGADocument5 pages2005 errepasoFORMULAZEZORGAJohn Cremer AceroNo ratings yet
- Konposatu Bitarrak Batera-ErantzunakDocument2 pagesKonposatu Bitarrak Batera-ErantzunakKoldo PZNo ratings yet
- DENAK - TALDETAN FormulazioaDocument8 pagesDENAK - TALDETAN FormulazioafloresdeNo ratings yet
- Elementu Eta Konposatu KimikoakDocument8 pagesElementu Eta Konposatu KimikoakEJAIRAM5No ratings yet
- Formulazio Ez-Organikoa DBH4Document10 pagesFormulazio Ez-Organikoa DBH4NaiaNo ratings yet
- Kimika PartzialaDocument1 pageKimika PartzialaJavier MartinezNo ratings yet
- FORMULAZIO EZ-ORGANIKOA - Documentos de GoogleDocument50 pagesFORMULAZIO EZ-ORGANIKOA - Documentos de GoogleAner Labaka UgarteNo ratings yet
- Formulazioa2 ErantzunakDocument2 pagesFormulazioa2 ErantzunakByLanderGmzNo ratings yet
- ParaEmail 22-23 FORMULAZIO EzORGANIKOA 1NNZZDocument2 pagesParaEmail 22-23 FORMULAZIO EzORGANIKOA 1NNZZaritz gonzalezNo ratings yet
- Ariketak (Hidroxidoak)Document1 pageAriketak (Hidroxidoak)anderguti2008No ratings yet
- Ariketak 1 (Emaitzak)Document5 pagesAriketak 1 (Emaitzak)d.laraNo ratings yet
- Formulazio Ez Org Azterketa EreduaDocument2 pagesFormulazio Ez Org Azterketa EreduatxoniasierNo ratings yet
- 3.ebal. FormulazioaDocument3 pages3.ebal. Formulazioaagurtzane.iturbeNo ratings yet
- Formulazio Ariketak (2) ZUZENKETAKDocument3 pagesFormulazio Ariketak (2) ZUZENKETAKPaula GimenoNo ratings yet
- FORMULAZIO EZORGANIKOA - BirpasoaDocument2 pagesFORMULAZIO EZORGANIKOA - BirpasoaIñaki Zarate GonzalezNo ratings yet
- Formulazioa Stock, SistematikoaDocument8 pagesFormulazioa Stock, SistematikoapixputNo ratings yet
- Microsoft Word - Formulazioa LaburpenaDocument1 pageMicrosoft Word - Formulazioa LaburpenaKoldo PZNo ratings yet
- Formulazio EzorganikoaDocument14 pagesFormulazio EzorganikoaJara EspumosaNo ratings yet
- Formulazio Azterketa (1) EmaitzekinDocument2 pagesFormulazio Azterketa (1) EmaitzekinIker Añibarro de CossíoNo ratings yet
- Nahastuta 2 ZuzendutaDocument3 pagesNahastuta 2 ZuzendutavitoNo ratings yet
- 1Document1 page1Vbg DaNo ratings yet
- Ariketak 1Document6 pagesAriketak 1d.laraNo ratings yet
- Nahasturik 3 ZuzendutaDocument2 pagesNahasturik 3 ZuzendutavitoNo ratings yet
- Formulazio AZTERKETA (1-A) 2018-2019Document2 pagesFormulazio AZTERKETA (1-A) 2018-2019Marcos PérezNo ratings yet
- Taula Dena ZuzendutaDocument13 pagesTaula Dena ZuzendutaDonaNo ratings yet
- 2 AltzairugintzaDocument41 pages2 AltzairugintzaAritz UgarteNo ratings yet
- Erreakzio KimikoakDocument3 pagesErreakzio KimikoakapartiedaNo ratings yet
- Ezorganikoa Konposatu HirutarrakDocument16 pagesEzorganikoa Konposatu HirutarrakXABIER JIMÉNEZ CARDONo ratings yet
- Hidruro BitarrakDocument2 pagesHidruro Bitarrakmaiteuranga98No ratings yet