Professional Documents
Culture Documents
CSIR NET June 2021 Physical
Uploaded by
Sankar Adhikari0 ratings0% found this document useful (0 votes)
81 views45 pagesCSIR NET June 2021 Physical Chemistry
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCSIR NET June 2021 Physical Chemistry
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
81 views45 pagesCSIR NET June 2021 Physical
Uploaded by
Sankar AdhikariCSIR NET June 2021 Physical Chemistry
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 45
Question ID: - 590
By reading the accompanying graph,
determine the INCORRECT statement out of
the following.
(1) Melting point increases with pressure
(2) Melting point decreases with pressure
(3) Boiling point increases with pressure
(4) Solid, liquid and gas can co-exist at the
same pressure and temperature
Question ID:-721
The penetrating power (R) and ionizing power (I) of α, β, and γ rays
follow the ordering
(1) 𝑅𝛽 > 𝑅𝛾 > 𝑅𝛼 𝑎𝑛𝑑 𝑙𝛽 > 𝑙𝛾 > 𝑙𝛼
(2) 𝑅𝛾 > 𝑅𝛽 > 𝑅𝛼 𝑎𝑛𝑑 𝑙𝛽 > 𝑙𝛾 > 𝑙𝛼
(3) 𝑅𝛽 > 𝑅𝛼 > 𝑅𝛾 𝑎𝑛𝑑 𝑙𝛼 > 𝑙𝛽 > 𝑙𝛾
(4) 𝑅𝛾 > 𝑅𝛽 > 𝑅𝛼 𝑎𝑛𝑑 𝑙𝛼 > 𝑙𝛽 > 𝑙𝛾
Question ID:-739
The miller indices of the planes parallel to the b axis and intersecting
the a and c axis, as shown in the figure, are
(1) (i) 101, (ii) 102
(2) (i) 102, (ii) 101
(3) (i) 100, (ii) 101
(4) (i) 100, (ii) 102
Question ID:-744
When yellow phosphorous is converted to red phosphorous, the
entropy and volume of the system do not change. The order of this
phase transition is most likely to be
(1) 3
(2) 2
(3) 1
(4) 0
Question ID:-745
When three of the phases of a two component system are
simultaneously in equilibrium the number of degrees of freedom is
(1) 0
(2) 1
(3) 2
(4) 3
Question ID:-794
Plutonium (atomic mass = 244 𝑔 𝑚𝑜𝑙 −1 ) crystallizes in monoclinic
lattice (a = 620 𝑚 ; 𝑏 480 𝑝𝑚; 𝑐 = 1100 𝑝𝑚 𝐵 = 102°) with 16
atoms per unit cell. The density in 𝑔 𝑐𝑚−3 will be close to (Use
𝑠𝑖𝑛 𝛽 = 0.98, 𝑠𝑖𝑛 𝛽/2 = 0.78)
(1) 25.38
(2) 16.12
(3) 12.69
(4) 20.26
Question ID:-797
For a weak electrolyte such as acetic acid, the relation among conductance
𝜆 , equilibrium constant 𝐾 and concentration 𝐶 can be expressed as:
(𝜆° 𝑖𝑠 𝑡ℎ𝑒 𝑐𝑜𝑛𝑑𝑢𝑐𝑡𝑎𝑛𝑐𝑒 𝑎𝑡 𝑖𝑛𝑓𝑖𝑛𝑖𝑡𝑒 𝑑𝑖𝑙𝑢𝑡𝑖𝑜𝑛)
1 1 𝐶𝜆
(1) = 0−
𝜆 𝜆 𝑘𝜆0
1 1 𝐶𝜆
(2) = 0−
𝜆 𝜆 𝑘𝜆02
1 1 𝐶𝜆
(3) = −
𝜆0 𝜆 𝑘𝜆02
1 𝐶𝜆
(4) = 02
𝜆 𝑘𝜆
Question ID: - 798
0 0
For the cell 𝐶𝑑 𝐶𝑑𝐶𝑙2 𝐴𝑔𝐶𝑙 𝐴𝑔; 𝐸𝑐𝑒𝑙𝑙 = 0.675 𝑉 𝑎𝑛𝑑 𝑑𝐸𝑐𝑒𝑙𝑙 /𝑑𝑇 =
− 6.5 × 10−4 𝑉𝐾 −1 at 27°𝐶.The ∆𝐻 𝑘𝐽 𝑚𝑜𝑙 −1
value for the reaction 𝐶𝑑 + 2𝐴𝑔𝐶𝑙 → 2𝐴𝑔 + 𝐶𝑑𝐶𝑙2 is closes to:
(1) -168
(2) -123
(3) -95
(4) -23
Question ID:-803
The molecule that will not absorb in the microwave region, but will
absorb in the infrared is
(1) 𝑁2
(2) 𝐶2 𝐻2
(3) 𝐻𝐶𝑙
(4) 𝐻2 𝑂
Question ID: - 804
The correct statements from the following set (i) to (iv) is
(i) If 𝑞 is the displacement from equilibrium for harmonic motion, the
potential energy is proportional to 𝑞.
(ii) If the vibrational frequency (𝑣) of HCI is 2990 𝑐𝑚−1 , its zero point
energy will be 1495 𝑐𝑚−1
(iii) The correct order of vibrational frequency of 𝑂- 1𝐻 𝑋1 , 0- 2𝐻 (𝑋2 ) and
𝑂- 3𝐻(𝑋3 )), is 𝑋2 > 𝑋2 > 𝑋3 .
(iv) The fundamental vibrational transition of a diatomic molecule appears at
1880 𝑐𝑚−1 . its first overtone will be at 940 𝑐𝑚−1
(1) i, ii, iii only
(2) i, ii, iii, iv
(3) ii, iii, only
(4) i, ii, iv only
Question ID:-808
The maximum number of phases that can be simultaneously in
equilibrium for a one component system is
(1) 1
(2) 2
(3) 3
(4) 4
Question ID: - 737
The combination of two reflections, 𝜎𝑣′ 𝜎𝑣′′ about an intersecting
mirror plane is equivalent to
(1) 𝑆𝑛
(2) 𝐶𝑛
(3) 𝜎ℎ
(4) 𝑖
Question ID: - 738
For a micro-canonical system, the correct probability distribution
function for energy is given by
(1) Exponential distribution function
(2) Gaussian distribution function
(3) Poisson distribution function
(4) Uniform distribution function
Question ID:-740
The volume of nitrogen gas adsorbed at STP to form a monolayer on
a porous solid surface is 22.4 𝑐𝑚3 𝑔−1 . If the area occupied by one
nitrogen gas molecule is 16.2Å2 , then the surface area (in 𝑐𝑚2 𝑔−1 )
of the solid is close to:
(1) 1.2 × 107
(2) 9.8 × 105
(3) 1.2 × 105
(4) 9.8 × 108
Question ID:-741
The amount of Ba 𝑁𝑂3 2 (molecular weight 261.32 amu) required to
be added to 500 g of a 0.11𝑚𝑜𝑙 𝑘𝑔−1 solution of 𝐾𝑁𝑂3 in order to
raise its ionic strength to 1.00 is approximately:
(1) 38.8 g
(2) 19.4 g
(3) 76.2 g
(4) 126.5 g
Question ID:-742
ො 𝑝ො𝑥2 ] is equivalent to
The commutator, [𝑥,
(1) −2𝑖ℎ𝑝ො𝑥
(2) 2𝑖ℎ𝑝ො𝑥
(3) −𝑖ℎ𝑝ො𝑥
(4) 𝑖ℎ𝑝ො𝑥
Question ID: - 747
The total 𝜋 -electron density on the four carbon atoms of trans
butadiene are in the ratio
(1) 1:1:1:1
(2) 1:2:2:1
(3) 1: 2 : 2 :1
(4) 1:3:3:1
Question ID: - 748
The reactive cross section is expected to be the largest for the
reaction
(1) 𝐿𝑖 + 𝐶𝑙2 → 𝐿𝑖𝐶𝑙 + 𝐶𝑙
(2) 𝑁𝑎 + 𝐶𝑙2
(3) 𝐾 + 𝐶𝑙2
(4) 𝑅𝑏 + 𝐶𝑙2
Question ID:-749 −
1
The rate of decomposition
− of a gas is 10 𝑚𝑀 𝑠 when 10 % is
1
reacted and it is 5 𝑚𝑀 𝑠 when 40% is reacted. The order of the
reaction is:
(1) 2
(2) 1.71
(3) 0
(4) 2.15
Question ID: - 750
For a person weighing 70 kg the minimal volume (in mL) of a fatal
dose of a compound with LD50 = 80 𝑚𝑔. 𝑘𝑔−1 , and density =
1.45 𝑔. 𝑚𝐿−1 is
(1) 5.6
(2) 3.9
(3) 0.8
(4) 0.4
Question ID:-755
Consider following terms. Identify those which are relevant to d.c.
polarography
A. Thermal current B. Supporting electrolyte C. Depolarization
D. Gelatin
Correct answer is
(1) A, B and C (2) A, B and D
(3) B, C and D (4) C and D only
Question ID: - 791
For every atom that is not shifted under 𝐶4 and 𝜎 symmetry
operation, the characters are, respectively,
(1) -1, -1
(2) 0, 0
(3) 1, 1
(4) -1, 1
Question ID:-792
The equivalent symmetry operation for 𝑆63 and 𝑆36 are, respectively,
(1) 𝐶3 𝑎𝑛𝑑 𝐶2
(2) 𝜎ℎ 𝑎𝑛𝑑 𝑖
(3) 𝜎ℎ 𝑎𝑛𝑑 𝐸
(4) 𝑖 𝑎𝑛𝑑 𝐸
Question ID: - 793
A system consists of N identical distinguishable non-interacting particles,
each having only two energy levels 0 and e. The expression of the heat
capacity at constant volume (𝐶𝑣 ) is given by (𝛽 = 1/𝑘𝐵𝑇 )
(1) 𝑁𝑘𝐵
∈𝛽 2
(2) 𝑁𝑘𝐵
1+𝑒 ∈𝛽
∈𝛽𝑒 ∈𝛽/2
(3) 𝑁𝑘𝐵
1+𝑒 ∈𝛽
2
∈𝛽𝑒 −2∈𝛽
(4) 𝑁𝑘𝐵
1+𝑒 −∈𝛽
Question ID:-795
In a Langmuir-type adsorption, a solid adsorbs 0.25 mg of a gas when
the pressure of the gas is 50 bar and 0.2 mg of the gas at 20 bar
pressure. The percentage of surface coverage at 50 bar is close to:
(1) 75
(2) 38
(3) 57
(4) 83
Question ID:-796
A sample of polystyrene is composed of three weight fractions: 0.20,
0.50 and 0.30. The molecular weight of these fractions are 10,000 ,
40,000 and 60,000, respectively. The weight average molecular
weight of this sample is:
(1) 40000
(2) 55000
(3) 50000
(4) 60000
Question ID: - 799
The rate constant for the reaction, 𝐴2 𝐵4 𝑂 → 𝐴𝐵4 + 𝐴𝑂, is described
10000 𝐾
as, log 𝑘 = 14.1 −
𝑇
The activation energy for this reaction (in KJ 𝑚𝑜𝑙 −1 ) is cosest to
(1) 191.4
(2) 83.14
(3) 382.8
(4) 166.28
Question ID:-800
For the reaction,
the equilibrium constant is 0.16 and 𝑘1 𝑖𝑠 3.3𝑥10−4 𝑠 −1 . The experiment is
started with pure cis form. The time taken for half the equilibrium amount of
trans isomer to be formed is about
(1) 290 s
(2) 580 s
(3) 190 s
(4) 480 s
Question ID:-801
The stopping potential for photoelectrons emitted from a surface
illuminated by light of frequency 6.0 × 108 𝑀𝐻𝑧 𝑖𝑠 0.72 𝑉. When the
incident frequency is changed, the stopping potential is found to be 1.44 V.
The new frequency is approximately (𝑒/ℎ = 2.4 × 1014 𝐶 𝐽−1 𝑠 −1 )
(1) 7 × 108
(2) 4 × 108
(3) 2 × 109
(4) 7 × 1014
Question ID:-802
Consider an electron 𝑚𝑒 = 9.1 × 10−31 𝑘𝑔 having energy 13.6 eV,
confined in an infinite potential well. If the potential energy inside
the well is zero, the expectation value for the square of the electron
speed, 𝑣 2 , is
(1) 3 × 1012 𝑚2 𝑠 −2
(2) 4.3 × 10−18 𝑚2 𝑠 −2
(3) 4.7 × 1012 𝑚2 𝑠 −2
(4) 4.7 × 1031 𝑚22 𝑠 −2
Question ID: - 805
The following data is obtained for a light diatomic (AB) molecule from
its rotational Raman spectrum.
𝐵 = 2𝑐𝑚−1 ; 𝑥𝑒 = 0.01; 𝑣𝑒 = 1600 𝑐𝑚−1 .
(1) 18348, 18356, 18368, 18380, 18388
(2) 18412, 18420, 18432, 18444, 18452
(3) 18380, 18388, 18400, 18412, 18420
(4) 18416, 18424, 18430, 18440, 18452
Question ID:-806
Liquid A has half the surface tension and twice the density of liquid B
at 30 °C. The contact angles of A and B are the same. If A rises 10 cm
in a capillary then the rise (in cm) of liquid B in the same capillary at
the same temperature will be equal to
(1) 60
(2) 10
(3) 40
(4) 20
Question ID:-807
The surface tension of a dilute soap solution is lower than that of pure
water because
(1) soap molecules accumulate more at the surface than in the bulk
solution
(2) soap molecules accumulate more in the bulk of the solution than on
the surface
(3) the soap molecules aggregate uniformly in the bulk and the surface.
(4)soap molecules form micellar structures at low concentration.
Question ID:-810
When a hydrogen atom is exposed to a perturbation V = E.z, the
first order correction to the wave function comes only from the
orbital
(1) 2s
(2) 2𝑝𝑧
(3) 3𝑝𝑦
(4) 3𝑑𝑧2
Happy Learning!
IFAS
India’s No.1 Institute for
CSIR NET | GATE | IIT JAM | TIFR | BARC | SET | PSC
| PhD Entrance |
91722 66888
You might also like
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Quantitative Aptitude Tricks - PDF Download: ImplificationDocument12 pagesQuantitative Aptitude Tricks - PDF Download: ImplificationSwapnarani JadavNo ratings yet
- How To Solve Clocks Questions Easily - PrepInstaDocument4 pagesHow To Solve Clocks Questions Easily - PrepInstaSankar AdhikariNo ratings yet
- Formulas For Clocks Questions - PrepInstaDocument3 pagesFormulas For Clocks Questions - PrepInstaSankar AdhikariNo ratings yet
- Fehling's Solution Is A Chemical Reagent Used To Differentiate BetweenDocument2 pagesFehling's Solution Is A Chemical Reagent Used To Differentiate BetweenSankar AdhikariNo ratings yet
- Math FundasDocument60 pagesMath FundasSankar AdhikariNo ratings yet
- Clock TricksDocument7 pagesClock TricksSankar AdhikariNo ratings yet
- CSIR NET June 2021 InorganicDocument37 pagesCSIR NET June 2021 InorganicSankar AdhikariNo ratings yet
- Master Quantitative Aptitude with tips & tricksDocument24 pagesMaster Quantitative Aptitude with tips & tricksSankar Adhikari0% (1)
- DICE - Verbal Reasoning QuestionsDocument13 pagesDICE - Verbal Reasoning QuestionsSankar AdhikariNo ratings yet
- 100 Shortcuts To Quantitative Aptitude Speed MattersDocument102 pages100 Shortcuts To Quantitative Aptitude Speed Mattersbest commentator barack100% (3)
- 30 of The Most Common Grammatical Errors We All Need To Stop MakingDocument19 pages30 of The Most Common Grammatical Errors We All Need To Stop MakingSankar AdhikariNo ratings yet
- Schematic representation of numbers on a dieDocument13 pagesSchematic representation of numbers on a dieSankar AdhikariNo ratings yet
- Troubleshooting Workup TricksDocument10 pagesTroubleshooting Workup TricksSankar AdhikariNo ratings yet
- CSIR NET June 2021 Organic ChemistryDocument99 pagesCSIR NET June 2021 Organic ChemistrySankar AdhikariNo ratings yet
- Quenching PyrophoricsDocument3 pagesQuenching PyrophoricsSankar AdhikariNo ratings yet
- Synthesis of Vinyl Ketones Via Mannich Bases: Was Nu: (Enolate)Document1 pageSynthesis of Vinyl Ketones Via Mannich Bases: Was Nu: (Enolate)Sankar AdhikariNo ratings yet
- H NMR Problem-Solving StrategiesDocument1 pageH NMR Problem-Solving StrategiesSankar AdhikariNo ratings yet
- Common MistakesDocument3 pagesCommon MistakesSankar AdhikariNo ratings yet
- Aromatic TM Skeleton NotesDocument7 pagesAromatic TM Skeleton NotesSankar AdhikariNo ratings yet
- Dr. Laurie S. Starkey, Cal Poly Pomona - NMR Spectroscopy: Spin-Spin CouplingDocument1 pageDr. Laurie S. Starkey, Cal Poly Pomona - NMR Spectroscopy: Spin-Spin CouplingSankar AdhikariNo ratings yet
- Aromatic TM Homework CHM 4220 Organic Synthesis, Dr. Laurie S. StarkeyDocument1 pageAromatic TM Homework CHM 4220 Organic Synthesis, Dr. Laurie S. StarkeySankar AdhikariNo ratings yet
- IRcorrelation TableDocument1 pageIRcorrelation TableSankar AdhikariNo ratings yet
- Mass Spec NotesDocument6 pagesMass Spec NotesSankar AdhikariNo ratings yet
- Organometallics CH 8 NotesDocument11 pagesOrganometallics CH 8 NotesSankar AdhikariNo ratings yet
- Introduction to NMR SpectroscopyDocument1 pageIntroduction to NMR SpectroscopySankar AdhikariNo ratings yet
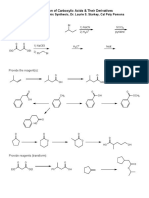
- Preparation of Carboxylic Acids & Their Derivatives: 1) MG 2) Co 3) H O 1) Nacn 2) H O Socl PyridineDocument1 pagePreparation of Carboxylic Acids & Their Derivatives: 1) MG 2) Co 3) H O 1) Nacn 2) H O Socl PyridineSankar AdhikariNo ratings yet
- sp21 234 r10 Extra Problems Organometallics KeyDocument8 pagessp21 234 r10 Extra Problems Organometallics KeySankar AdhikariNo ratings yet
- Preparation of Ethers & Amines: 1) Nah 2) NaomeDocument1 pagePreparation of Ethers & Amines: 1) Nah 2) NaomeSankar AdhikariNo ratings yet
- Ace General Chemistry 1 and 2Document187 pagesAce General Chemistry 1 and 2Ari Singh100% (2)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Dorf Chapter 11Document16 pagesDorf Chapter 11ekasNo ratings yet
- Maintenance Plant Balance Sections 5-6 Scrubber and Grinding Mill Equipment SelectionDocument22 pagesMaintenance Plant Balance Sections 5-6 Scrubber and Grinding Mill Equipment SelectionJr.JeanNo ratings yet
- GLOBE PFG-red planetary geared vane motors for industrial applicationsDocument26 pagesGLOBE PFG-red planetary geared vane motors for industrial applicationsTarcio TomNo ratings yet
- Scientific Communication-Bsc I Year Old Questions2076 - Khulla NoteDocument1 pageScientific Communication-Bsc I Year Old Questions2076 - Khulla Noteankishshrestha8No ratings yet
- B2 1-B2 1M-2009PVDocument7 pagesB2 1-B2 1M-2009PVRobert Johnson14% (7)
- SprinkCALC III Report - Bld7.Riser1Document30 pagesSprinkCALC III Report - Bld7.Riser1Igor CvijovicNo ratings yet
- Applications of Duality Theory (Diewert)Document9 pagesApplications of Duality Theory (Diewert)Franco BailonNo ratings yet
- Calculation of Fin Efficiency For Wet and Dry Fins.Document16 pagesCalculation of Fin Efficiency For Wet and Dry Fins.Wilfredo Ruiz100% (1)
- Steel Making Axle Forging Heat Treatment MachiningDocument41 pagesSteel Making Axle Forging Heat Treatment MachiningAsif Ali PCNo ratings yet
- Brønsted-Lowry Theory of Acids and BasesDocument4 pagesBrønsted-Lowry Theory of Acids and Basescayla mae carlosNo ratings yet
- Belzona 1121: Product Specification SheetDocument2 pagesBelzona 1121: Product Specification SheetQuy RomNo ratings yet
- Verify Geometrically That c×a+b = c×a + c×b Using ParallelogramsDocument4 pagesVerify Geometrically That c×a+b = c×a + c×b Using ParallelogramsblehboNo ratings yet
- Write Up On LPBP SystemDocument9 pagesWrite Up On LPBP SystemKhushboo PandeyNo ratings yet
- Bare Overhead Transmission Conductors ": Selection and Application"Document23 pagesBare Overhead Transmission Conductors ": Selection and Application"Adnan KhanNo ratings yet
- Generator Details ReportDocument1 pageGenerator Details Reportwaseem kausarNo ratings yet
- Piping Design GuideDocument65 pagesPiping Design GuideShrey PatelNo ratings yet
- Q3 Science 5 Periodical Test Questions No HeadingDocument4 pagesQ3 Science 5 Periodical Test Questions No HeadingWea Joy Mantolino-MasNo ratings yet
- Calorie Food Samples HighestDocument2 pagesCalorie Food Samples HighestAndrea KingNo ratings yet
- A10 A Pid Va 718602 201Document1 pageA10 A Pid Va 718602 201zhangNo ratings yet
- 3G3mx2-v2 Ds e 1 1 csm1126864Document54 pages3G3mx2-v2 Ds e 1 1 csm1126864Pertti HänninenNo ratings yet
- FND GFM 13725098Document133 pagesFND GFM 13725098sanjayprakash1979No ratings yet
- Lockout Tagout LOTO Catalogue enDocument44 pagesLockout Tagout LOTO Catalogue enAlphaNo ratings yet
- ITTC - Recommended Procedures and Guidelines: Experimental Wake Scaling MethodsDocument8 pagesITTC - Recommended Procedures and Guidelines: Experimental Wake Scaling MethodsajayNo ratings yet
- Foundation Calculation Sheet: Title DescriptionDocument10 pagesFoundation Calculation Sheet: Title DescriptionrobianggaNo ratings yet
- Electromagnetism Course Notes (PHYS09060) 2017/18Document98 pagesElectromagnetism Course Notes (PHYS09060) 2017/18KeKa TVNo ratings yet
- Ch02-Zealey PRE 4thppDocument67 pagesCh02-Zealey PRE 4thppMuhammad Kashif RashidNo ratings yet
- Chilled Water Pipe DESIGNDocument22 pagesChilled Water Pipe DESIGNTanjim FakirNo ratings yet
- 7 Things That Affect Your Vibration Frequency From The Point of View of Quantum PhysicsDocument12 pages7 Things That Affect Your Vibration Frequency From The Point of View of Quantum PhysicsLluisNo ratings yet
- Direct drive disperser guideDocument2 pagesDirect drive disperser guideAlvaro Nerviani AltieriNo ratings yet
- Pos FormatDocument6 pagesPos FormatChelmarie CuracheaNo ratings yet