Professional Documents
Culture Documents
Gas Law Formula
Uploaded by
Dana Fuentes0 ratings0% found this document useful (0 votes)
13 views1 pageCopyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
13 views1 pageGas Law Formula
Uploaded by
Dana FuentesCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
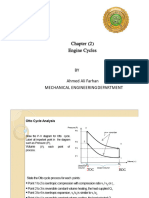
Gas Law Formula
Gas Law Formula
Boyle’s Law P1 V 1=P2 V 2
pressure & volume
P2 V 2 P2 V 2 - inversely
P1= V 1= proportional
V1 P1
P1 V 1 P1 V 1
P2= V 2=
V2 P2
Charles’ Law V1 V2
= volume & temp.
T1 T2
- directly
V 2T 1 V 1T 2 proportional
V 1= T 1=
T2 V2
V 1T 2 V 2T 1
V 2= T 2=
T1 V1
Gay-Lussac’s Law P2 T 1 P1 T 2
P1 = T 1= pressure & temp.
T2 P2
- directly
P1 T 2 P2T 1 proportional
P2 = T 2=
T1 P1
P 1 P2
=
T1 T 2
Combined Law P1V 1 P2V 2
=
T1 T2
Ideal Gas Law PV =nT (0.0821)
V = volume in dm3 P = pressure in kPa R = ideal gas constant
T = temperature in Kelvin n = number of moles R = 0.0821 atm∙L/n∙K
You might also like
- Gas LawsDocument2 pagesGas Lawskimtaemin1997.stageNo ratings yet
- Formelsammlung - ZustandsänderungenDocument2 pagesFormelsammlung - ZustandsänderungenTrần Nguyên KhôiNo ratings yet
- Ideal GasDocument1 pageIdeal GasMike Raphy T. VerdonNo ratings yet
- KinjutsuDocument21 pagesKinjutsuLocus Jhun MichaelNo ratings yet
- CH 2 PDFDocument34 pagesCH 2 PDFkrishnaNo ratings yet
- Scroll of Seals 1Document25 pagesScroll of Seals 1Anthony MacalindongNo ratings yet
- Thermodynamic Processes and DerivationDocument10 pagesThermodynamic Processes and DerivationAbenayaNo ratings yet
- Formulario Termodinamica 1Document2 pagesFormulario Termodinamica 1Jesus LyroyNo ratings yet
- استنتاج2Document30 pagesاستنتاج2mohamed orifNo ratings yet
- Ideal and Real GasesDocument8 pagesIdeal and Real GasesAnand_N_GNo ratings yet
- Gas Laws Problems WorksheetDocument3 pagesGas Laws Problems WorksheetMimimiyuhNo ratings yet
- Gas Laws Problems Worksheet PDFDocument3 pagesGas Laws Problems Worksheet PDFBinu Kumar S0% (1)
- Gas Laws Problems WorksheetDocument3 pagesGas Laws Problems WorksheetWoodland ElvesNo ratings yet
- Lecture 2 EDocument8 pagesLecture 2 EMihai MirceaNo ratings yet
- Gas LawsDocument6 pagesGas LawskimNo ratings yet
- Thermodynamics FormulaDocument9 pagesThermodynamics FormulaJayvie TumangNo ratings yet
- Old Thermo Exam Formula SheetDocument1 pageOld Thermo Exam Formula SheetjonahNo ratings yet
- Unit 2Document42 pagesUnit 2Kalai Selvan BaskaranNo ratings yet
- 2 Really An Ideal Gases!Document14 pages2 Really An Ideal Gases!GajiniNo ratings yet
- 211 ch3 (Part 2)Document39 pages211 ch3 (Part 2)محمد فاضلNo ratings yet
- 2.gaseous State PDFDocument13 pages2.gaseous State PDFP. E. I. AcademicsNo ratings yet
- Formula Closed SystemDocument1 pageFormula Closed SystemPANKWORLDNo ratings yet
- Gaseous State Worksheet PDFDocument10 pagesGaseous State Worksheet PDFHarsh Agarwal100% (1)
- A V P A V P: PressureDocument2 pagesA V P A V P: PressurePearl Alexandra FabitoNo ratings yet
- Thermodynamics FormulasDocument1 pageThermodynamics FormulasDarshan V SimsonNo ratings yet
- Thermodynamics FormulasDocument1 pageThermodynamics FormulasIshani SantraNo ratings yet
- Genchem FormulasDocument2 pagesGenchem FormulasAliance Mary AdorioNo ratings yet
- Change The % Into Grams (23% Na - 23g Na) : Grams of An Element Total MolesDocument2 pagesChange The % Into Grams (23% Na - 23g Na) : Grams of An Element Total MolesAliance Mary AdorioNo ratings yet
- 2001 SAT Practice TestDocument2 pages2001 SAT Practice TestAnonymous 23uIgnINo ratings yet
- Lecture Notes On Compressor 2019 PDFDocument21 pagesLecture Notes On Compressor 2019 PDFJerome MaldaNo ratings yet
- Penurunan Persamaan KontinuitasDocument1 pagePenurunan Persamaan KontinuitasDila DirgahaditpaNo ratings yet
- LS1 - Determination of Jet Velocity and Nozzle EfficiencyDocument9 pagesLS1 - Determination of Jet Velocity and Nozzle EfficiencyfaezahjalalNo ratings yet
- Thermodynamics CH 5Document32 pagesThermodynamics CH 5WILYNo ratings yet
- Week 1 Science Module 4th QDocument6 pagesWeek 1 Science Module 4th QAyow MendozaNo ratings yet
- Scroll of Seals 2Document11 pagesScroll of Seals 2Anthony MacalindongNo ratings yet
- The Seven Generals Process N P-V-T H U W W Q S Isothermal T C 1Document1 pageThe Seven Generals Process N P-V-T H U W W Q S Isothermal T C 1Anthony Macalindong100% (2)
- Hydro 1 Module 2Document13 pagesHydro 1 Module 2Jericho Alfred Rullog Sapitula100% (1)
- 102MAE Thermodynamics Formula SheetDocument2 pages102MAE Thermodynamics Formula SheetBogdan ProfirNo ratings yet
- Q W U H S: Isotérmico DT 0 Isobárico DP 0 Isocórico DV 0 Adiabático DQ 0Document2 pagesQ W U H S: Isotérmico DT 0 Isobárico DP 0 Isocórico DV 0 Adiabático DQ 0Aldasaurio SPNo ratings yet
- Chapter 13 GasesDocument30 pagesChapter 13 GasesGwen100% (1)
- Kinetic Theory of GasesDocument51 pagesKinetic Theory of GasesKamatchi MNo ratings yet
- Entropy View of Theoretical Processes .Document11 pagesEntropy View of Theoretical Processes .Muket AgmasNo ratings yet
- Solution & Colligative Properties Complete Chapter Notes For IITDocument40 pagesSolution & Colligative Properties Complete Chapter Notes For IITNazmer XNo ratings yet
- E Rathakrishnan Gas Dynamics SolutionsDocument216 pagesE Rathakrishnan Gas Dynamics SolutionsVigneshVickey67% (15)
- Plane Wall 1-D Steady State Conduction W/O Heat GenerationDocument1 pagePlane Wall 1-D Steady State Conduction W/O Heat GenerationI Gede Darma SusilaNo ratings yet
- Formulario Termo UpbDocument1 pageFormulario Termo UpbDivad_28No ratings yet
- All Formulas Revision in One Shot For JEE Main 2021 Feb Attempt (21-02-2021)Document85 pagesAll Formulas Revision in One Shot For JEE Main 2021 Feb Attempt (21-02-2021)Kampili akashNo ratings yet
- Termodinamica ch03Document35 pagesTermodinamica ch03Rebeca AlmeidaNo ratings yet
- Gas Laws Problems WorksheetDocument3 pagesGas Laws Problems WorksheetLeonardo SierraNo ratings yet
- BSLTPCH 3 P 3 B13Document2 pagesBSLTPCH 3 P 3 B13اركان دحام سويد محمدNo ratings yet
- ThermodynamicsDocument13 pagesThermodynamicsMonique OrugaNo ratings yet
- 05 States of Matter Formula Sheets QuizrrDocument10 pages05 States of Matter Formula Sheets QuizrrIshita AgarwalNo ratings yet
- States of Matter Formula Sheet @cbseinfiniteDocument8 pagesStates of Matter Formula Sheet @cbseinfiniteSulveNo ratings yet
- Expansions, Energy, Enthalpy Isothermal Gas Expansion: P V T P V TDocument7 pagesExpansions, Energy, Enthalpy Isothermal Gas Expansion: P V T P V TNaveen NaviNo ratings yet
- 12 InvertersDocument17 pages12 InvertersElectrical EngineeringNo ratings yet
- 12 InvertersDocument17 pages12 InvertersБатсайхан ДугаржавNo ratings yet
- Me 6404 - Thermal Engineering UNIT - IV - Air CompressorDocument34 pagesMe 6404 - Thermal Engineering UNIT - IV - Air CompressorJay Mark CayonteNo ratings yet
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet