Professional Documents
Culture Documents
Periodic Trends Lab
Uploaded by
YellowOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Periodic Trends Lab
Uploaded by
YellowCopyright:
Available Formats
Name: Muhammad Shaaf Partner: Alejandro Date: 10/14/22
Title: Periodic Trends
Purpose: To explore the reactivity trends of metals in groups and periods of the periodic table.
Materials: Test tubes, Calcium, Sodium, Potassium, Magnesium, Zinc, Tin, Silicon, Graduated cylinder, water,
HCI, scale, dropper.
Procedure (Numbered):
Part 1
1. Watch the demonstration of the following demonstration of the following elements being placed in
water. Record your observations. a. Calcium b. Sodium c. Potassium lace a small piece of Magnesium in
a test tube and cover it with water. Record your observation
Part 2
1. Dump the water out of the test tube containing the Mg (from Part 1) but keep the Mg in the test
tube. 2. Obtain a small piece of Zinc and Tin. 3. Place each metal in a separate test tube. 4. Using
a dropper, add a small amount of HCl (approximately 2mL) to each test tube, just enough the
sample. 5. Record your observations in the data table.
Part 3
1. Determine the density of Silicon by finding its mass and volume. 2. Create your own data table below
and record your data.
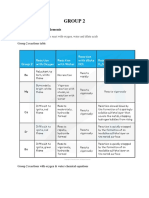
Data and Analysis:
Part 1
Metal Group observation
Calcium 2 Bubbles, Fizzes up
Sodium 1 Fizzes up, sparks and smokes
Potassium 1 Sparks and smokes immediately
Magnesium 2 It fizzes
Part 2
Metal Group observation
Magnesium 2 More fizzing than water, still
slow
Zinc 12 Fizzes up, all bubbly
Tin 14 Nothing
Part 3
Metal Estimated Density Density (g/ml)
Silicon 2.0 g 1.2
Estimate of silicon’s density (g/ml): 1.2 g/ml
Discussion (Questions):
1. That metal reactivity increases down groups and decreases from left to right across periods
2. Metal reactivity increases down groups.
3. Metal reactivity decreases from left to right across periods
Conclusion:
Part 1: 1. Potassium
2. difference in stability of their electron configurations as atoms and as ions.
Part 2: 1. Zinc
2. Sn____Mg_____Zn_______Ca____Na_____k
Least reactive ---------------------------------Most reactive
Explanation: When tin was put in HCI it did next to nothing, while Magnesium Fizzed slowly, Zinc fizzed up
almost instantly and got very bubbly, Calcium fizzed up more than Zinc, Sodium sparked and smoked in the
water, and Potassium immediately started sparking and smoking
Part 3: 1. My result does support my original estimate because I estimated 2 g/ml and the result was 1.2 g/ml.
You might also like
- Group 2 ElementsDocument9 pagesGroup 2 ElementsSumaya TraoreNo ratings yet
- Reactivity 3Document20 pagesReactivity 3Iyanuoluwa AdebayoNo ratings yet
- 2.2. Group 2, The Alkaline Earth MetalsDocument2 pages2.2. Group 2, The Alkaline Earth Metalshaseeb3382786No ratings yet
- Alkali MetalsDocument7 pagesAlkali Metalsokguserfucker idontgiveashitNo ratings yet
- Group I & IIDocument3 pagesGroup I & IINoor Ul AinNo ratings yet
- Chemistry Topic 4 KODocument2 pagesChemistry Topic 4 KOOmar AmancioNo ratings yet
- 10 Cie Group 2Document6 pages10 Cie Group 2Anshu MovvaNo ratings yet
- 2.2 Revision Guide Group 2 AqaDocument4 pages2.2 Revision Guide Group 2 AqaVivehaNo ratings yet
- Group 2Document31 pagesGroup 2Shima SenseiiNo ratings yet
- Analysis of IonsDocument2 pagesAnalysis of IonsPaarth BansalNo ratings yet
- Chapter 8 Periodic TableDocument9 pagesChapter 8 Periodic TablenothingisfingnothingNo ratings yet
- 3.1 The Reactivity Series of MetalsDocument17 pages3.1 The Reactivity Series of MetalsWafa OsmanNo ratings yet
- CAIE Chemistry A-Level: 10: Group 2Document6 pagesCAIE Chemistry A-Level: 10: Group 2ahumanbeinginearthNo ratings yet
- g10 Chem ETT APR 2023 REVISION GUIDEDocument10 pagesg10 Chem ETT APR 2023 REVISION GUIDEHa Khanh Ngoc TranNo ratings yet
- FaziraRazak Group IIADocument58 pagesFaziraRazak Group IIAaieyinHengNo ratings yet
- 3 1 2 Group 2Document2 pages3 1 2 Group 2DecklinNo ratings yet
- CIE Chemistry A Level: 10: Group 2Document7 pagesCIE Chemistry A Level: 10: Group 2Shujaat SiddiquiNo ratings yet
- 353CH81 Day IiDocument10 pages353CH81 Day IiMarwan FarhanNo ratings yet
- Group 2 Part 1 EdexcelDocument3 pagesGroup 2 Part 1 EdexcelKevin The Chemistry TutorNo ratings yet
- Detailed Notes Topic 4 Inorganic Chemistry and The Periodic Table Edexcel Chemistry A LevelDocument13 pagesDetailed Notes Topic 4 Inorganic Chemistry and The Periodic Table Edexcel Chemistry A LevelttjjjNo ratings yet
- CIE Chemistry A Level: 10: Group 2Document7 pagesCIE Chemistry A Level: 10: Group 2Mildred MunatsiNo ratings yet
- 11 - Group 2Document37 pages11 - Group 2enderothNo ratings yet
- Period 3 Elements ExperimentDocument2 pagesPeriod 3 Elements ExperimentjenniferNo ratings yet
- Cambridge Book Group 2Document8 pagesCambridge Book Group 2Aree WonNo ratings yet
- Group II ElementsDocument15 pagesGroup II ElementsDoveNo ratings yet
- Laboratory Redox Reaction: Gsci1103L-General Chemistry 1 LabDocument5 pagesLaboratory Redox Reaction: Gsci1103L-General Chemistry 1 LabAndrea AurielleNo ratings yet
- CHEM111-Experiment No 2Document5 pagesCHEM111-Experiment No 2ryalphawolfNo ratings yet
- Chemistry Form 5 KSSM: 8 February 2021Document24 pagesChemistry Form 5 KSSM: 8 February 2021NurNo ratings yet
- Group 1 & 2 MetalsDocument8 pagesGroup 1 & 2 MetalsDaniel BerryNo ratings yet
- Analyzing Group 1 ElementDocument19 pagesAnalyzing Group 1 ElementAlia PixieNo ratings yet
- Activity Series Lab (Akey)Document4 pagesActivity Series Lab (Akey)jcastill77No ratings yet
- Unit 6 EM Theory Book 1 (Group 1-15) v23.0Document20 pagesUnit 6 EM Theory Book 1 (Group 1-15) v23.0Thilanka LiyanageNo ratings yet
- Chemistry Grade 11 Chapter VIIIDocument44 pagesChemistry Grade 11 Chapter VIIIJ.K HomerNo ratings yet
- Reactions PDFDocument6 pagesReactions PDFAnshu MovvaNo ratings yet
- Reactivity Series Worksheet QuDocument8 pagesReactivity Series Worksheet Quقاتل مستأجرNo ratings yet
- Chapter 3 Periodic OxfordDocument18 pagesChapter 3 Periodic OxfordEyad ELshenawyNo ratings yet
- Group 2 12 STEM 2 October 13, 2017 MATIENZO, GabrielDocument4 pagesGroup 2 12 STEM 2 October 13, 2017 MATIENZO, GabrielgabNo ratings yet
- CHEM SPM Chapter 4 Periodic Tble TeacherDocument24 pagesCHEM SPM Chapter 4 Periodic Tble Teacherangie0812No ratings yet
- Reaction of Metals With OxygenDocument9 pagesReaction of Metals With Oxygenmanery23No ratings yet
- Group 2 MetalsDocument19 pagesGroup 2 MetalsSelena JayyNo ratings yet
- NCERT Solutions For Class 10 March 29 Science Chapter 3 Metals and Non MetalsDocument11 pagesNCERT Solutions For Class 10 March 29 Science Chapter 3 Metals and Non Metalsarvinda1981No ratings yet
- Group 2 Part 2 EdexcelDocument2 pagesGroup 2 Part 2 EdexcelKevin The Chemistry TutorNo ratings yet
- 3 1 2 Group 2Document2 pages3 1 2 Group 2Garret GordonNo ratings yet
- Apchemistrylab1 4Document3 pagesApchemistrylab1 4api-263752592No ratings yet
- Metals and Non-Metals NotesDocument18 pagesMetals and Non-Metals NotesAzeem IqbalNo ratings yet
- 2.6 NotesDocument6 pages2.6 NotesLisa DentonNo ratings yet
- Metals and Non-Metals NotesDocument18 pagesMetals and Non-Metals NotesMustafa Khan100% (1)
- It's Ability of Atom in Covalent Molecule To Attract Electrons of The Bond TowardsDocument2 pagesIt's Ability of Atom in Covalent Molecule To Attract Electrons of The Bond Towardsmido titoNo ratings yet
- Metals and Non-Metals Notes - RemovedDocument15 pagesMetals and Non-Metals Notes - RemovedCyber Atharv100% (1)
- Chemistry Notes (Metals)Document4 pagesChemistry Notes (Metals)Teo Jia Ming Nickolas67% (3)
- Worksheet 2Document1 pageWorksheet 2Alicia VallaNo ratings yet
- NCERT Solutions For CBSE Class 10 Science Chapter 3 Metals and Non MetalsDocument10 pagesNCERT Solutions For CBSE Class 10 Science Chapter 3 Metals and Non MetalsHari PrasadNo ratings yet
- Group 2Document19 pagesGroup 2Muhammad KalimNo ratings yet
- Expt. 4 Galvanic Corrosion GROUP 2Document6 pagesExpt. 4 Galvanic Corrosion GROUP 2John Carlo BuscadoNo ratings yet
- 8B Group 1 2Document14 pages8B Group 1 2pediaNo ratings yet
- SinglereplacementrxnlabDocument3 pagesSinglereplacementrxnlabapi-239309345No ratings yet
- 3 NOV Class 10 Metals and Non-Metals ChemDocument40 pages3 NOV Class 10 Metals and Non-Metals Chemgourav kaliaNo ratings yet
- Chapter 14.1: Metals Introduction.: How Are The Properties of Metals Related To Their Structure?Document5 pagesChapter 14.1: Metals Introduction.: How Are The Properties of Metals Related To Their Structure?Lixue's PoyaiNo ratings yet
- How Do Bases React With Metals?: Activity 2.3Document1 pageHow Do Bases React With Metals?: Activity 2.3sciencee2009No ratings yet
- Scanned DocumentsDocument1 pageScanned DocumentsYellowNo ratings yet
- Ain't I A Woman Rhetorical AnalysisDocument1 pageAin't I A Woman Rhetorical AnalysisYellowNo ratings yet
- Final Essay - EditedDocument2 pagesFinal Essay - EditedYellowNo ratings yet
- Scanned DocumentsDocument1 pageScanned DocumentsYellowNo ratings yet
- Untitled Document - EditedDocument1 pageUntitled Document - EditedYellowNo ratings yet
- Untitled Document - EditedDocument2 pagesUntitled Document - EditedYellowNo ratings yet
- Formative Thesis Statement and Evidence-1Document2 pagesFormative Thesis Statement and Evidence-1YellowNo ratings yet
- Unipoxy LiningDocument3 pagesUnipoxy LiningDien Thoai Nguyen HuuNo ratings yet
- IWA SG Global TrendsDocument113 pagesIWA SG Global Trendsandik_yNo ratings yet
- Prevention: Cutting DryersDocument5 pagesPrevention: Cutting DryersAljay ImperialNo ratings yet
- Universidad Tecnologica Centroamericana: Cambios de FaseDocument14 pagesUniversidad Tecnologica Centroamericana: Cambios de FaseAndrea SortoNo ratings yet
- SEPTAGE COLLECTION CALCULATION and SEPTAGE TREATMENT PLANT DESIGN CONCEPTDocument31 pagesSEPTAGE COLLECTION CALCULATION and SEPTAGE TREATMENT PLANT DESIGN CONCEPTKladees WorldNo ratings yet
- Material Safety Data Sheet Avashalestop/ActDocument4 pagesMaterial Safety Data Sheet Avashalestop/Actfs1640No ratings yet
- Correlation Between Prostate Cancer and ArsenicDocument26 pagesCorrelation Between Prostate Cancer and ArsenicCharlie BravoNo ratings yet
- Thermal Energy Analysis Using TRNSYS in PCM Storage TankDocument5 pagesThermal Energy Analysis Using TRNSYS in PCM Storage TankAnonymous lPvvgiQjRNo ratings yet
- Convotherm Oes 6.10 y 20.20 Service HandbookDocument285 pagesConvotherm Oes 6.10 y 20.20 Service HandbookAlbert PL CSTNo ratings yet
- Catalogo - Amerec 2013Document28 pagesCatalogo - Amerec 2013Ruben VidalNo ratings yet
- Visualizing Sustainability Performance of Manufacturing Systems Using Sustainable Value Stream Mapping (Sus-VSM)Document11 pagesVisualizing Sustainability Performance of Manufacturing Systems Using Sustainable Value Stream Mapping (Sus-VSM)jeffersonNo ratings yet
- Comparison of Three Methods For Natural Gas DehydrationDocument6 pagesComparison of Three Methods For Natural Gas Dehydrationalbert_ben13No ratings yet
- Selina Solutions For Class 9 Physics Chapter 6 Heat and EnergyDocument22 pagesSelina Solutions For Class 9 Physics Chapter 6 Heat and EnergyAnubrata SarkarNo ratings yet
- The Importance of Water For Our LifeDocument7 pagesThe Importance of Water For Our LifeBillie0% (1)
- WellsDocument25 pagesWellsTHEJSWINo ratings yet
- Physics 5054: MCM Nkana Secondary School End of Topic Test 1Document10 pagesPhysics 5054: MCM Nkana Secondary School End of Topic Test 1Jedediah PhiriNo ratings yet
- Terms of Reference For Environmental Impact Assessment of IndustriesDocument28 pagesTerms of Reference For Environmental Impact Assessment of Industrieskrishan lalNo ratings yet
- Aqua Reslin SuperDocument4 pagesAqua Reslin SuperSyed Sajid KamalNo ratings yet
- Koolman R410A IOM 2020Document40 pagesKoolman R410A IOM 2020JX SNo ratings yet
- Perrustol Vno New - eDocument2 pagesPerrustol Vno New - eNghia Phan TrungNo ratings yet
- Topic1-1 Thermal PrincipleDocument40 pagesTopic1-1 Thermal PrincipleEdith Carumbana JusayanNo ratings yet
- Sustainable Urban Drainage-Green RoofDocument36 pagesSustainable Urban Drainage-Green RoofGabriel WongNo ratings yet
- Topic Outline Flood ControlDocument6 pagesTopic Outline Flood ControlCathy AnchetaNo ratings yet
- Type Series Booklet: Submersible Borehole PumpDocument104 pagesType Series Booklet: Submersible Borehole PumpJosko SanticNo ratings yet
- Operator'S Manual: CLP-5024 CLP-7034Document86 pagesOperator'S Manual: CLP-5024 CLP-7034Cesar Eugenio Sanhueza ValdebenitoNo ratings yet
- NRAC Publication No. 170 An Introduction To Water Chemistry in Freshwater AquacultureDocument4 pagesNRAC Publication No. 170 An Introduction To Water Chemistry in Freshwater AquacultureSk RajNo ratings yet
- Geothermal For Electrical PowerDocument26 pagesGeothermal For Electrical PowerOfyan HavanaNo ratings yet
- Presentation of Environmental Science.: Topic: Environmental Pollution "BBA-IV"Document16 pagesPresentation of Environmental Science.: Topic: Environmental Pollution "BBA-IV"Hammad HassanNo ratings yet
- SPE-WVS-215: A Methodology For Lab-Stimulation Tests To Improve Well ProductionDocument20 pagesSPE-WVS-215: A Methodology For Lab-Stimulation Tests To Improve Well ProductionRaifel MoralesNo ratings yet
- Suplayer DsDocument93 pagesSuplayer Dskuncibalipinangsia2023No ratings yet