Professional Documents
Culture Documents
Classifying & Balancing Chemical Reactions Name
Uploaded by
Monette CabugayanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Classifying & Balancing Chemical Reactions Name
Uploaded by
Monette CabugayanCopyright:
Available Formats
Classifying & Balancing Chemical Reactions Name:
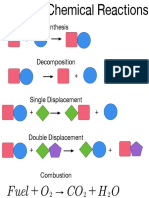
Synthesis SY
Balance each chemical reaction below and identify the reaction Decomposition DC
Combustion C
type by writing the appropriate abbreviation from the box.
Single Displacement SD
Double Displacement DD
Acid-Base AB
Balance Type
1) HBr + Mg(OH)2 MgBr2 + H2O ________________
2) PbBr2 + HCl HBr + PbCl2 ________________
3) CoBr3 + CaSO4 CaBr2 + Co2(SO4)3 ________________
4) C4H12 + O2 CO2 + H2O ________________
5) B2O3 + H2O H3BO3 ________________
6) H2O H2 + O2 ________________
7) C2H4 + O2 CO2 + H2O ________________
8) Mg + Fe2O3 MgO + Fe ________________
9) H3PO4 + Ca(OH)2 Ca3(PO4)2 + H2O ________________
10) Cl2 + KBr KCl + Br2 ________________
11) H3AsO4 As2O5 + H2O ________________
12) S8 + O2 SO3 ________________
13) Bi(NO3)3 + Al2(SO4)3 Bi2(SO4)3 + Al(NO3)3 _______________
14) HgO Hg + O2 ________________
15) P + O2 P2O3 ________________
16) HBr + Ba(OH)2 BaBr2 + H2O ________________
17) Fe + H2O Fe3O4 + H2 ________________
18) C3H8 + O2 H2O + CO2 ________________
Classifying & Balancing Chemical Reactions Name: Answer Key
Synthesis SY
Balance each chemical reaction below and identify the reaction Decomposition DC
Combustion C
type by writing the appropriate abbreviation from the box.
Single Displacement SD
Double Displacement DD
Acid-Base AB
Balance Type
1) 2 HBr + 1 Mg(OH)2 1 MgBr2 + 2 H2O _____AB_________
2) 1 PbBr2 + 2 HCl 2 HBr + 1 PbCl2 _____DD________
3) 2 CoBr3 + 3 CaSO4 3 CaBr2 + 1 Co2(SO4)3 _____DD________
4) 1 C4H12 + 7 O2 4 CO2 + 6 H2O _____C__________
5) 1 B2O3 + 3 H2O 2 H3BO3 _____SY_________
6) 2 H2O 2 H2 + 1 O2 _____DC_________
7) 1 C2H4 + 3 O2 2 CO2 + 2 H2O _____C__________
8) 3 Mg + 1 Fe2O3 3 MgO + 2 Fe _____SD_________
9) 2 H3PO4 + 3 Ca(OH)2 1 Ca3(PO4)2 + 6 H2O _____AB_________
10) 1 Cl2 + 2 KBr 2 KCl + 1 Br2 _____SD_________
11) 2 H3AsO4 1 As2O5 + 3 H2O _____DC_________
12) 1 S8 + 12 O2 8 SO3 _____SY_________
13) 2 Bi(NO3)3 + 1 Al2(SO4)3 1 Bi2(SO4)3 + 2 Al(NO3)3 ____DD________
14) 2 HgO 2 Hg + 1 O2 _____DC_________
15) 4P + 3 O2 2 P2O3 _____SY_________
16) 2 HBr + 1 Ba(OH)2 1 BaBr2 + 2 H2O _____AB_________
17) 3 Fe + 4 H2O 1 Fe3O4 + 4 H2 _____SD_________
18) 1 C3H8 + 5 O2 4 H2O + 3 CO2 _____C__________
You might also like
- Naming Mixed Ionic and Covalent CompoundsDocument1 pageNaming Mixed Ionic and Covalent Compoundsapi-325791445No ratings yet
- Unit 1 Worksheet Packet KEY Name Period Worksheet 1 (Goals 1 - 6) SECTION 2.1 PROPERTIES of MATTER (Pages 34 - 37)Document11 pagesUnit 1 Worksheet Packet KEY Name Period Worksheet 1 (Goals 1 - 6) SECTION 2.1 PROPERTIES of MATTER (Pages 34 - 37)wendzNo ratings yet
- 3 - Hybridization WorksheetDocument2 pages3 - Hybridization Worksheetkomal sheikh0% (1)
- Counting Atoms WorksheetDocument3 pagesCounting Atoms WorksheetDeysi LopezNo ratings yet
- Separate mixtures using techniques like distillation and chromatographyDocument5 pagesSeparate mixtures using techniques like distillation and chromatographydanielmahsaNo ratings yet
- 4500-NH3 NITROGEN (AMMONIA) DISTILLATIONDocument10 pages4500-NH3 NITROGEN (AMMONIA) DISTILLATIONdjainikurniyawan_47No ratings yet
- WORKSHEET (Chemical Equations) PDFDocument4 pagesWORKSHEET (Chemical Equations) PDFnobodyNo ratings yet
- Chemistry Worksheet: Matter #1Document6 pagesChemistry Worksheet: Matter #1Anisah MahmudahNo ratings yet
- Quiz 2 - Measurements and DensityDocument4 pagesQuiz 2 - Measurements and DensityCarolyn CampitaNo ratings yet
- Worksheet 1.1 Intermolecular Forces: Liquids, Solids, and Phase ChangesDocument2 pagesWorksheet 1.1 Intermolecular Forces: Liquids, Solids, and Phase ChangesRisciella 18No ratings yet
- Marymount International School Second Semester Examination Grade 10 ChemistryDocument10 pagesMarymount International School Second Semester Examination Grade 10 Chemistryrawan alkaisiNo ratings yet
- Experiment RedoxDocument6 pagesExperiment RedoxJaaizah JaafarNo ratings yet
- Examview 2009 2010 Sch4c Unit 3 (Org) Test Exam PrepDocument4 pagesExamview 2009 2010 Sch4c Unit 3 (Org) Test Exam Prepabebaw matebuNo ratings yet
- Common Chemical Reactions in Everyday Life: ImagewillbeuploadedsoonDocument3 pagesCommon Chemical Reactions in Everyday Life: ImagewillbeuploadedsoonMei NalunneNo ratings yet
- 060 Half Life WorksheetDocument3 pages060 Half Life WorksheetLin Xian XingNo ratings yet
- Diagnostic Test in General Chemistry 1Document13 pagesDiagnostic Test in General Chemistry 1Dearest Notes100% (1)
- 3.3 (B) Mole N MassDocument20 pages3.3 (B) Mole N MassFidree AzizNo ratings yet
- Reviewer in General Chemistry 2Document77 pagesReviewer in General Chemistry 2Ana Marie100% (1)
- Governor's Hills Science School Chemistry ExamDocument3 pagesGovernor's Hills Science School Chemistry ExamAriane DionisioNo ratings yet
- Lab 8 CHM130LL Identification of Cations and AnionsDocument6 pagesLab 8 CHM130LL Identification of Cations and AnionsFatimah AzzahrahNo ratings yet
- Elements, Compounds and MixturesDocument4 pagesElements, Compounds and MixturesFatema KhatunNo ratings yet
- Bonding Test ReviewDocument5 pagesBonding Test ReviewRonaldo ManaoatNo ratings yet
- Types of Chemical Reaction Quiz (Worksheet)Document2 pagesTypes of Chemical Reaction Quiz (Worksheet)yaoi yuriNo ratings yet
- Isomer WorksheetDocument3 pagesIsomer Worksheetronnie schwiersNo ratings yet
- Sci 6 Suspensions and ColloidsDocument29 pagesSci 6 Suspensions and ColloidsDionelyn CruzNo ratings yet
- 21 Types of Chemical Reactions-SDocument6 pages21 Types of Chemical Reactions-SMichael BensonNo ratings yet
- Practice Exam 2 ChemistDocument5 pagesPractice Exam 2 ChemistFATIN FARHANAH BINTI HALIDIN MoeNo ratings yet
- Nuclear Power Debate Project PacketDocument6 pagesNuclear Power Debate Project Packetapi-252900678No ratings yet
- Gen SpbobincompletedomDocument3 pagesGen Spbobincompletedomapi-259614222No ratings yet
- Mole Conversion ClassworkDocument4 pagesMole Conversion ClassworkAdvanced PastryNo ratings yet
- Formula Mass WorksheetDocument2 pagesFormula Mass WorksheetJha DBestNo ratings yet
- Homework 6.1 Balancing Chemical ReactionsDocument2 pagesHomework 6.1 Balancing Chemical ReactionsDorothy CastilloNo ratings yet
- Flame Test LabDocument5 pagesFlame Test LabRaman Aylur SubramanianNo ratings yet
- Oxidation ReductionDocument47 pagesOxidation ReductionAbdulraqeb AlawadhiNo ratings yet
- Ionic Packet For Lab Chem 2010 2011Document16 pagesIonic Packet For Lab Chem 2010 2011Marianne Garcia50% (2)
- Physical and Chemical Properties of MatterDocument1 pagePhysical and Chemical Properties of Matterriza amoresNo ratings yet
- Organic Compounds PropertiesDocument3 pagesOrganic Compounds Propertiesgloria tolentinoNo ratings yet
- Worksheet - Classification of Matter - KeyDocument1 pageWorksheet - Classification of Matter - KeyVaughnNo ratings yet
- Writing Formulas and Naming Molecular CompoundsDocument2 pagesWriting Formulas and Naming Molecular Compoundsplt2010100% (1)
- Properties of Liquids and Intermolecular ForcesDocument5 pagesProperties of Liquids and Intermolecular ForcesJohnnard BelenNo ratings yet
- Common Names and Formulas of Important Chemical CompoundsDocument7 pagesCommon Names and Formulas of Important Chemical Compoundsayush singhNo ratings yet
- Covalent Compound Worksheet SolutionsDocument2 pagesCovalent Compound Worksheet SolutionsDVRao100% (1)
- Acids and Bases QuizDocument6 pagesAcids and Bases Quizleah rualesNo ratings yet
- Vsepr HandoutDocument2 pagesVsepr Handout20718 LAY BUFFON FERNANDO GROSSONo ratings yet
- Third Periodical Examination Chemistry I 2011-2012Document9 pagesThird Periodical Examination Chemistry I 2011-2012Rogelio PontejoNo ratings yet
- Classify Chemical and Physical ChangesDocument6 pagesClassify Chemical and Physical ChangesEnael FernandezNo ratings yet
- Redox ReactionsDocument4 pagesRedox Reactionsmahika gaurNo ratings yet
- Acid Base WorksheetDocument2 pagesAcid Base WorksheetBobby Barnes100% (2)
- 1 ElectronConfigurationspacket PTDocument8 pages1 ElectronConfigurationspacket PTEsmeralda ConradNo ratings yet
- (CHEM 108) M1C1 - Introduction To Chemistry-Matter and MeasurementDocument29 pages(CHEM 108) M1C1 - Introduction To Chemistry-Matter and MeasurementVladimir TimbrezaNo ratings yet
- Johniya Cochran - Ionic and Covalent Bonds ActivityDocument4 pagesJohniya Cochran - Ionic and Covalent Bonds ActivityJohniya CochranNo ratings yet
- Word Equations Worksheet Chemical ReactionsDocument2 pagesWord Equations Worksheet Chemical ReactionsAffan PresentationsNo ratings yet
- Chemical Formulas All WorksheetsDocument19 pagesChemical Formulas All Worksheetsshivam33% (3)
- SOLVED MULTIPLE CHOICE QUESTIONS ON CHEMICAL EQUILIBRIUMDocument12 pagesSOLVED MULTIPLE CHOICE QUESTIONS ON CHEMICAL EQUILIBRIUMApeksha Garg100% (2)
- Precipitation ReactionsDocument3 pagesPrecipitation ReactionsborgiamatriceNo ratings yet
- Worksheet Percent CompositionDocument2 pagesWorksheet Percent CompositionNkemzi Elias NzetengenleNo ratings yet
- Classifying Matter and Energy StatesDocument7 pagesClassifying Matter and Energy StatesJam Uly GastyNo ratings yet
- Periodic Trends WorksheetDocument4 pagesPeriodic Trends WorksheetMahmoud AladdasiNo ratings yet
- Science 9: The Variety of Carbon CompoundsDocument10 pagesScience 9: The Variety of Carbon Compoundsrussel castilloNo ratings yet
- Models of Molecular Compounds Lab (Ms. Possible)Document5 pagesModels of Molecular Compounds Lab (Ms. Possible)Steven GomescoelloNo ratings yet
- Empirical and Molecular Formula WorksheetDocument3 pagesEmpirical and Molecular Formula WorksheetAgee AbdullaNo ratings yet
- A Voyage Through EquationsDocument14 pagesA Voyage Through Equationsshakira100% (1)
- ReactionTimeLab 1Document6 pagesReactionTimeLab 1Monette CabugayanNo ratings yet
- 2 Weeks: Bell Ringer JournalDocument9 pages2 Weeks: Bell Ringer JournalMonette CabugayanNo ratings yet
- + Synthesis: Fuel O CO HODocument1 page+ Synthesis: Fuel O CO HOMonette CabugayanNo ratings yet
- Measurements, Math and Estimation - English/Metric Conversion (Exotic Units)Document15 pagesMeasurements, Math and Estimation - English/Metric Conversion (Exotic Units)Monette CabugayanNo ratings yet
- Synthesis Decomposition: Teacher InstructionsDocument5 pagesSynthesis Decomposition: Teacher InstructionsMonette CabugayanNo ratings yet
- Types of Chemical ReactionsDocument35 pagesTypes of Chemical ReactionsJemina R. B. EspedillonNo ratings yet
- Quantum Number and Electron Orbital Review GameDocument3 pagesQuantum Number and Electron Orbital Review GameMonette CabugayanNo ratings yet
- PEH Periodic Table (Principles) - Get The Table Organized in Time! Lab Manual (English)Document6 pagesPEH Periodic Table (Principles) - Get The Table Organized in Time! Lab Manual (English)Monette CabugayanNo ratings yet
- iHP Vs VHPDocument1 pageiHP Vs VHPErwin WinNo ratings yet
- The Periodic Table of ElementsDocument41 pagesThe Periodic Table of ElementsPawan GoswamiNo ratings yet
- Hemical Ndustries Ewsletter: CEH Marketing Research Report AbstractDocument13 pagesHemical Ndustries Ewsletter: CEH Marketing Research Report AbstractRicardo Javier PlasenciaNo ratings yet
- MT - 01 PCM JM Paper (26.06.2022) 12thDocument22 pagesMT - 01 PCM JM Paper (26.06.2022) 12thAnurag PatelNo ratings yet
- Polypropylene - WikipediaDocument17 pagesPolypropylene - WikipediaMohsin KhanNo ratings yet
- Evers and Sons Inc.: Welding Procedure Specification (WpsDocument3 pagesEvers and Sons Inc.: Welding Procedure Specification (WpsRaja HoneNo ratings yet
- 1 IntroDocument55 pages1 IntroNivedhan GandhiNo ratings yet
- Removing Stains at HomeDocument15 pagesRemoving Stains at HomeAlex MontillaNo ratings yet
- Enzyme Reactions WorkshietDocument2 pagesEnzyme Reactions Workshietojamil2No ratings yet
- Part-C Defects - Nptel PDFDocument5 pagesPart-C Defects - Nptel PDFLakhwant Singh KhalsaNo ratings yet
- 9th Class Chemistry Guess PapersDocument11 pages9th Class Chemistry Guess PapersMarkpiciNo ratings yet
- Revision Notes On Acids, Bases and SaltsDocument3 pagesRevision Notes On Acids, Bases and SaltsVikas SharmaNo ratings yet
- Tarras-Wahlberg Et Al. (2001)Document23 pagesTarras-Wahlberg Et Al. (2001)Mariela Huaripata HuaripataNo ratings yet
- Vitec 1000Document1 pageVitec 1000eduardoNo ratings yet
- Chapter 21 Cutting Tools: MET 33800 Manufacturing ProcessesDocument25 pagesChapter 21 Cutting Tools: MET 33800 Manufacturing ProcessesAlissa Saphira PutriNo ratings yet
- CH CH: CH No CH No Oh NoDocument6 pagesCH CH: CH No CH No Oh NoIhsan MokhlisseNo ratings yet
- Setalux 1756 VV-65 TdsDocument2 pagesSetalux 1756 VV-65 TdsroybombomNo ratings yet
- High Temperature Plastics: Present by Vaishnavi SoneDocument8 pagesHigh Temperature Plastics: Present by Vaishnavi SoneNilanjana MishraNo ratings yet
- Specification For Carbon and Alloy Steel Nuts For Bolts For High-Pressure or High-Temperature Service, or BothDocument18 pagesSpecification For Carbon and Alloy Steel Nuts For Bolts For High-Pressure or High-Temperature Service, or BothPaulo SantosNo ratings yet
- Chapter8 PhaseDiagram HandoutsDocument27 pagesChapter8 PhaseDiagram Handoutswagdy87No ratings yet
- Oil & Gas Industry OverviewDocument98 pagesOil & Gas Industry OverviewEmmanuel ByensitaNo ratings yet
- Carbohydrate Qualitative TestsDocument16 pagesCarbohydrate Qualitative TestsJanNo ratings yet
- Welding Rod DetailsDocument4 pagesWelding Rod DetailsFernando RomeroNo ratings yet
- Parameterization of Arynophiles: Experimental Investigations To-Wards A Quantitative Understanding of Aryne Trapping ReactionsDocument8 pagesParameterization of Arynophiles: Experimental Investigations To-Wards A Quantitative Understanding of Aryne Trapping ReactionsavikcuiitkgpNo ratings yet
- ReportDocument21 pagesReportFaisal AkhterNo ratings yet
- Chrome General Surgical CatalogueDocument268 pagesChrome General Surgical CatalogueAli100% (1)
- V-20 VTK VarnishTestKitsDocument2 pagesV-20 VTK VarnishTestKitsEduardo CramerNo ratings yet
- Alccocrete (HS)Document2 pagesAlccocrete (HS)Siddhesh Kamat MhamaiNo ratings yet