Professional Documents
Culture Documents
9 - Les 3 Types Des Cristaux Ioniques
Uploaded by
Khalilou Klai0 ratings0% found this document useful (0 votes)
6 views1 pageOriginal Title
9_Les 3 types des cristaux ioniques
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views1 page9 - Les 3 Types Des Cristaux Ioniques
Uploaded by
Khalilou KlaiCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
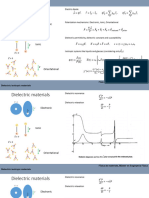
𝒓+
Calcul du rapport 𝒓−
a 𝟑 a 𝐚 𝟑
Contact cation-anion : 𝒓+ + 𝒓− = Contact cation-anion : 𝒓+ + 𝒓− = Contact cation-anion : 𝒓+ + 𝒓− =
𝟐 𝟐 𝟒
Contact anion-anion : a = 2 𝒓− Contact anion-anion : a 𝟐 = 4 𝒓− Contact anion-anion : a 𝟐 = 4 𝒓−
𝒓+ 𝒓+ 𝒓+ 𝟑
𝒓− = 𝟑 - 1 = 0,732 𝒓−
= 𝟐 - 1 = 0,414
𝒓−
= 𝟐
- 1 = 0,225
Coordinence [4/4] Coordinence [6/6] Coordinence [8/8] 𝒓+
Type ZnS Type NaCl Type CsCl 𝒓−
0,225 0,414 0,732
Tous droits réservés © TakiAcademy.com
You might also like
- LW09 Lead Lag ControlDocument11 pagesLW09 Lead Lag ControlRaveendra ReddyNo ratings yet
- FiltersDocument28 pagesFiltersFuture GamingNo ratings yet
- Complicated Rate Equations: 3.1 Integration of Rate EquationDocument1 pageComplicated Rate Equations: 3.1 Integration of Rate EquationDwi Ratna MustafidaNo ratings yet
- Electric PropertiesDocument36 pagesElectric PropertiessaraNo ratings yet
- Bevel GearsDocument2 pagesBevel GearsRonnieNo ratings yet
- Bevel Gears May SulatDocument2 pagesBevel Gears May SulatRonnieNo ratings yet
- 12th Physics 5 Mark Important Question Study Material Prepared by Mr. R SaravananDocument17 pages12th Physics 5 Mark Important Question Study Material Prepared by Mr. R SaravananN E X NexonNo ratings yet
- Electrical Simulation LabDocument51 pagesElectrical Simulation LabRithwakNo ratings yet
- Ch.4 Roots of PolynomialsDocument1 pageCh.4 Roots of Polynomialsstefanwaller812No ratings yet
- BJT Biasing Networks - Class - (No PNP)Document32 pagesBJT Biasing Networks - Class - (No PNP)thatokgosiyagae389No ratings yet
- Flow Chart For Flexure Design of T-Section BeamDocument2 pagesFlow Chart For Flexure Design of T-Section BeamArif Samoon100% (1)
- Chapter 2 - Lecture 3Document14 pagesChapter 2 - Lecture 3Muhammad HarithNo ratings yet
- Other RLC Resonant Circuits and Bode Plots 2024Document14 pagesOther RLC Resonant Circuits and Bode Plots 2024str0261No ratings yet
- Centroid and Moment of IntertiaDocument1 pageCentroid and Moment of Intertialyka deguzmanNo ratings yet
- First Order CircuitsDocument3 pagesFirst Order CircuitsEffecure HealthcareNo ratings yet
- Paralelo R1, R2, y R3 R9Document18 pagesParalelo R1, R2, y R3 R9Vladimir Moreno RiosNo ratings yet
- PVT Formula Cheat SheetDocument10 pagesPVT Formula Cheat SheetkamilkakarNo ratings yet
- Electric Circuit Analysis Project ReportDocument10 pagesElectric Circuit Analysis Project Reporta a a aNo ratings yet
- Questions On HW 1Document47 pagesQuestions On HW 1Abdullah IshaqNo ratings yet
- Nominal Rate of Interest v.01Document13 pagesNominal Rate of Interest v.01FSR Uwu2419No ratings yet
- 12 Simple Notes (EM) - 5 MarksDocument16 pages12 Simple Notes (EM) - 5 MarksNidhiNo ratings yet
- Corpo Fiche Mid TermDocument2 pagesCorpo Fiche Mid Termjuliettemrtn1No ratings yet
- Submitted To: Sir. Permanand SootharDocument10 pagesSubmitted To: Sir. Permanand SootharAmeer JaanNo ratings yet
- Conjugate Beam MethodDocument6 pagesConjugate Beam MethodArjie RecarialNo ratings yet
- Wisnu Adi Nugroho: A New Method To Estimate Gas Kick Rising Velocity in Oil Base MudDocument1 pageWisnu Adi Nugroho: A New Method To Estimate Gas Kick Rising Velocity in Oil Base Mudadi nugrohoNo ratings yet
- N (Electron Concentration)Document4 pagesN (Electron Concentration)HameedullahNo ratings yet
- Magnetic Effect of Current - Quick Revision - Classnotes (For Print Out) - (Lakshya JEE 2023)Document8 pagesMagnetic Effect of Current - Quick Revision - Classnotes (For Print Out) - (Lakshya JEE 2023)MeghanshNo ratings yet
- Cajas 5Document1 pageCajas 5Naomi GonzálesNo ratings yet
- Problem 1Document7 pagesProblem 1Alphamae VicenteNo ratings yet
- Lembar Perhitungan ReagaenDocument8 pagesLembar Perhitungan ReagaenZahra AlifiaNo ratings yet
- Machine Model GENQECDocument9 pagesMachine Model GENQECManuelNo ratings yet
- Ionic Equilibria Class 12th Chemistry NotesDocument18 pagesIonic Equilibria Class 12th Chemistry Notesnazim92% (12)
- Thermal Lab I Equation SheetDocument1 pageThermal Lab I Equation SheetaldbasiqaisNo ratings yet
- Calculate Flow Rate Through Control Valve For Incompressible Fluids When There Is No Flow MeterDocument26 pagesCalculate Flow Rate Through Control Valve For Incompressible Fluids When There Is No Flow MeterHadi VeyseNo ratings yet
- Topics 2 Machine Elements ReviewerDocument8 pagesTopics 2 Machine Elements ReviewerOwel CabugawanNo ratings yet
- Thermodynamic Properties of FluidDocument9 pagesThermodynamic Properties of FluidZyber ColcolNo ratings yet
- Theory 1 - Theory of Structures 1: Module 6: Cables and ArchesDocument27 pagesTheory 1 - Theory of Structures 1: Module 6: Cables and ArchesAnfrett E. BanggollayNo ratings yet
- EE-215 Electronic Devices and Circuits Assignment#2Document5 pagesEE-215 Electronic Devices and Circuits Assignment#2umer luqmanNo ratings yet
- Microwave Resonators NotesDocument31 pagesMicrowave Resonators Notesameya1981No ratings yet
- Chapter #4#Document36 pagesChapter #4#fikadubiruk87No ratings yet
- 라그랑주의 연분수 정리 - 영어Document12 pages라그랑주의 연분수 정리 - 영어김정민No ratings yet
- FORMULASDocument4 pagesFORMULASirish cerezaNo ratings yet
- Cheat SheetDocument4 pagesCheat Sheeti 3l3jNo ratings yet
- Slides - Plug Flow Reactor (2018)Document36 pagesSlides - Plug Flow Reactor (2018)Meireza Ajeng Pratiwi100% (1)
- Havens2020 Article LinearFractionalTransformation PDFDocument17 pagesHavens2020 Article LinearFractionalTransformation PDFSandra NgNo ratings yet
- Maximum Power TransferDocument19 pagesMaximum Power TransferTuan LeNo ratings yet
- TRK Problem 8.3Document12 pagesTRK Problem 8.3Wahidin ShekoskiNo ratings yet
- Fibonacci SequenceDocument14 pagesFibonacci SequenceKyle GamutanNo ratings yet
- 기초회로이론 챕터4 과제Document54 pages기초회로이론 챕터4 과제lostdream0515No ratings yet
- 6CPT40 - Lecture 2 - Multicomponent Distillation & Rate Based Models-1Document80 pages6CPT40 - Lecture 2 - Multicomponent Distillation & Rate Based Models-1nagod22No ratings yet
- Exp 11 21110006 CL352Document7 pagesExp 11 21110006 CL352Abhinav AnandNo ratings yet
- 2.4 - HydraulicsDocument3 pages2.4 - HydraulicsFrancisco AriasNo ratings yet
- ppt-Chapter-1-Semiconductor Diodes & ApplicsDocument59 pagesppt-Chapter-1-Semiconductor Diodes & Applicsramya hegdeNo ratings yet
- Soal P8.26 Dan P13.2Document14 pagesSoal P8.26 Dan P13.2Jaka Septian KustantoNo ratings yet
- EE201 Ch5 Operational Amplifiers (Part 2) PDFDocument21 pagesEE201 Ch5 Operational Amplifiers (Part 2) PDFThịnh Nguyễn ViếtNo ratings yet
- LP Filter Aktif RA S1 DTE ITS PDFDocument19 pagesLP Filter Aktif RA S1 DTE ITS PDFHadi WidjajaNo ratings yet
- LP Filter Aktif RA S1 DTE ITS PDFDocument19 pagesLP Filter Aktif RA S1 DTE ITS PDFHadi WidjajaNo ratings yet