Professional Documents
Culture Documents
12th Chemistry Que Paper Design 2022-23
Uploaded by
ram vermaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
12th Chemistry Que Paper Design 2022-23
Uploaded by
ram vermaCopyright:
Available Formats
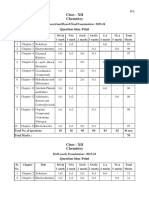
Question Paper Design
Subject : Chemistry Class : 12th Session : 2022-23
Paper : Annual or Supplementary Time : 3 Hrs. Marks : 70
1 Weightage to Objectives:
Objective K U A S Total
Percentage of Marks 40 30 20 10 100
Marks 28 21 14 07 70
2 Weightage to Form of Questions:
Form of Question E SA VSA Objective type Total
No of Question 03 08 08 01(15 parts) 20
Marks allotted 15 24 16 15 70
Estimated time (in 65 55 40 20 180
Min.)
3 Weightage to Content:
Reading Comprehension Marks
UNITS Marks No of Questions Total Marks
1. Unit:2- Solutions
2. Unit:3- Electro Chemistry
3. Unit:4- Chemical Kinetics
4. Unit:8- d & f Block Elements
5. Unit:9- Co-ordination Compounds
6. Unit:10- Halo Alkanes and Halo Arenes
7. Unit:11- Alcohols, Phenols and Ethers
8. Unit:12- Aldehydes, Ketones and Carboxylic Acids
9. Unit:13- Amines and Diazonium Salts
10. Unit:14- Biomolecules
Total
4 Scheme of Sections : A:B:C:D
5 Scheme of Sections : Internal Choice in long answer Questions i.e. essay type in two
Question.
6 Difficulty level : Difficult : 10% Marks
Average : 50% Marks
Easy : 40% Marks
------------------------------------------------------------------------------------------------- ------------
Abbreviations: K (Knowledge of element of language) C (Comprehension) E (Expression)
A (Appreciation) E(Essay Type) SA (Short Answer) VSA (Very Short Answer) O (Objective Type)
Note:- Unit:1 (Solid State), Unit:5 (Surface Chemistry), Unit:6 (General Principles &
Processes of Isolation of Element) Unit:7 (p-Block Elements) Unit:15 (Polymers)
Unit:16 (Chemistry in Every Day Life) are to be deleted for session2022-23.
You might also like
- NDA TemplateDocument8 pagesNDA TemplateCha AgaderNo ratings yet
- Chemistry 21 Set PDFDocument348 pagesChemistry 21 Set PDFNutzie Y80% (5)
- AccountingDocument20 pagesAccountingjanlaan0% (1)
- Paneer Tikka Masala RecipeDocument41 pagesPaneer Tikka Masala Recipeimemyself009No ratings yet
- Rate of Turn IndicatorDocument16 pagesRate of Turn IndicatorGlen Mac100% (1)
- Bety Garma Niños en Análisis - de La Exp - 20180427153854Document6 pagesBety Garma Niños en Análisis - de La Exp - 20180427153854pamelapsi100% (1)
- The Irresistible OfferDocument17 pagesThe Irresistible OfferAndrew YakovlevNo ratings yet
- Leeer para RepasarrrrrDocument21 pagesLeeer para RepasarrrrrAriana MeliánNo ratings yet
- Sterling Test Prep DAT General Chemistry Review: Complete Subject ReviewFrom EverandSterling Test Prep DAT General Chemistry Review: Complete Subject ReviewNo ratings yet
- SST Paper DesignDocument1 pageSST Paper DesignsunnyNo ratings yet
- UntitledDocument32 pagesUntitledDharshan Dharshan sdNo ratings yet
- Hindi Paper DesignDocument1 pageHindi Paper DesignsunnyNo ratings yet
- Physival Edu DesignDocument1 pagePhysival Edu DesignsunnyNo ratings yet
- a2819297c03c423e78d1d5b85479ed23Document16 pagesa2819297c03c423e78d1d5b85479ed23Techno ClubNo ratings yet
- Marks: Question Paper Design 2020-21Document1 pageMarks: Question Paper Design 2020-21Anuj SharmaNo ratings yet
- CHEMISTRY, PRACTICAL BASED COURSE, Distribution of Marks (OUT 0F 75) Is As FollowsDocument38 pagesCHEMISTRY, PRACTICAL BASED COURSE, Distribution of Marks (OUT 0F 75) Is As FollowsNampiNo ratings yet
- 10 Maths EM PDFDocument7 pages10 Maths EM PDFpradeep36No ratings yet
- Navneet Chemistry Question BankDocument34 pagesNavneet Chemistry Question BankshriNo ratings yet
- Facts, Syllabus and Pattern: What Is KVPY?Document20 pagesFacts, Syllabus and Pattern: What Is KVPY?Er Chinmoy NandaNo ratings yet
- VU CSE Syllabus Spring 20231Document77 pagesVU CSE Syllabus Spring 20231sabbir shohanNo ratings yet
- Maths EMDocument7 pagesMaths EMSubramanyam K.V.RNo ratings yet
- Physics XIAssessment Scheme 20222023Document24 pagesPhysics XIAssessment Scheme 20222023goacmkakaNo ratings yet
- JEE Main 2022 Analysis (June/1st Attempt)Document7 pagesJEE Main 2022 Analysis (June/1st Attempt)Resonance EduventuresNo ratings yet
- Basic Electrical Technology Course PlanDocument5 pagesBasic Electrical Technology Course PlanEzio AuditoreNo ratings yet
- Science Paper DesignDocument1 pageScience Paper DesignsunnyNo ratings yet
- Social EM.23Document11 pagesSocial EM.23Tharangini AkkinsNo ratings yet
- DCME - 1st Sem SyllabusDocument87 pagesDCME - 1st Sem Syllabusshashi preethamNo ratings yet
- CSE (Data Science) - R22-1st Year Course Structure & SyllabusDocument40 pagesCSE (Data Science) - R22-1st Year Course Structure & SyllabusKasani Tirumala tejaNo ratings yet
- D0685 ChemistryDocument34 pagesD0685 ChemistryShreyasNo ratings yet
- Class VIII ResonanceDocument13 pagesClass VIII Resonancebiren71No ratings yet
- Achievement Test Analysis - Class VI - Sonal JainDocument10 pagesAchievement Test Analysis - Class VI - Sonal JainabhayNo ratings yet
- Social Studies emDocument58 pagesSocial Studies emAkshit ReddyNo ratings yet
- JEE Main 2022 July Session 2 Shift-1 (DT 25-07-2022) Detailed AnalysisDocument7 pagesJEE Main 2022 July Session 2 Shift-1 (DT 25-07-2022) Detailed AnalysisResonance EduventuresNo ratings yet
- Diploma in Electrical Engineering C 18 SyllabusDocument89 pagesDiploma in Electrical Engineering C 18 Syllabuskiran045No ratings yet
- Social EMDocument11 pagesSocial EMSubramanyam K.V.RNo ratings yet
- JEE Main 2022 July Session 2 Shift-2 (DT 25-07-2022) Detailed AnalysisDocument7 pagesJEE Main 2022 July Session 2 Shift-2 (DT 25-07-2022) Detailed AnalysisResonance EduventuresNo ratings yet
- SCO 9TH CLASS Subjective Model WTS-02 QP 01-10-2022Document6 pagesSCO 9TH CLASS Subjective Model WTS-02 QP 01-10-2022ourganti08No ratings yet
- Chem 120 Fall 2012 PS1 Answer Key PDFDocument18 pagesChem 120 Fall 2012 PS1 Answer Key PDFSteven LyNo ratings yet
- Syllabus ChemDocument5 pagesSyllabus ChemDGA GAMINGNo ratings yet
- Mathematics Myp 5Document36 pagesMathematics Myp 5BRIGHTON ONYANGONo ratings yet
- Blue Print Yearly Vi To Viii 2022Document5 pagesBlue Print Yearly Vi To Viii 2022Nauhwar AbhiNo ratings yet
- 22MATE11Document5 pages22MATE11Gayatri JoshiNo ratings yet
- Chemistry Class XIIDocument4 pagesChemistry Class XIIshilpinathbhowmikNo ratings yet
- FE Final ExamDocument2 pagesFE Final Examabraham kassahunNo ratings yet
- DEIE - 1st Sem SyllabusDocument99 pagesDEIE - 1st Sem SyllabusSYNDICATE GamerNo ratings yet
- Distribution of Topics For Mid-Term, First-Term and Second-Term (From June 2016 0nwards) Subject: CHEMISTRYDocument5 pagesDistribution of Topics For Mid-Term, First-Term and Second-Term (From June 2016 0nwards) Subject: CHEMISTRYvishweshwara hollaNo ratings yet
- JEE Main 2022 Analysis (June/1st Attempt)Document7 pagesJEE Main 2022 Analysis (June/1st Attempt)Resonance EduventuresNo ratings yet
- 22MATC11Document5 pages22MATC11Akash GVNo ratings yet
- JEE (Advanced) 2014: A Detailed AnalysisDocument9 pagesJEE (Advanced) 2014: A Detailed AnalysisDivya ReddyNo ratings yet
- Chemistry SyllabusDocument6 pagesChemistry SyllabusDarshan ks GowdaNo ratings yet
- Basic Electronics ECT101 Course File - MNIT 23072019Document18 pagesBasic Electronics ECT101 Course File - MNIT 23072019Aryan KhanNo ratings yet
- JEE Main 2022 Analysis (June/1st Attempt)Document7 pagesJEE Main 2022 Analysis (June/1st Attempt)Resonance EduventuresNo ratings yet
- 22CHES12Document7 pages22CHES12The Golden Hacker'sNo ratings yet
- JEE Main 2023 April Session 2 Shift-2 (DT 08-04-2023) Detailed AnalysisDocument13 pagesJEE Main 2023 April Session 2 Shift-2 (DT 08-04-2023) Detailed AnalysisResonance EduventuresNo ratings yet
- FTHT A, Fa-I - : Session-2020-21 AnnualsupplementaryDocument1 pageFTHT A, Fa-I - : Session-2020-21 AnnualsupplementaryChemophilicNo ratings yet
- 3 0 3 MAT1002 None: MAT2002 Complex Variables and Partial Differential Equations 1.0Document2 pages3 0 3 MAT1002 None: MAT2002 Complex Variables and Partial Differential Equations 1.0Arjun ArjunNo ratings yet
- 01 - CHEM 102 Sample Midterm 2 QuestionsDocument10 pages01 - CHEM 102 Sample Midterm 2 QuestionsPallavi RawatNo ratings yet
- BP Science 6to8Document1 pageBP Science 6to8Naitik GoelNo ratings yet
- Learning Activity Statistics Exercises Template 1Document8 pagesLearning Activity Statistics Exercises Template 1Linghui Gao-GustaveNo ratings yet
- JEE Main 2023 April Session 2 Shift-1 (DT 08-04-2023) Detailed AnalysisDocument13 pagesJEE Main 2023 April Session 2 Shift-1 (DT 08-04-2023) Detailed AnalysisResonance EduventuresNo ratings yet
- JEE Main 2022 Analysis (June/1st Attempt)Document7 pagesJEE Main 2022 Analysis (June/1st Attempt)Resonance EduventuresNo ratings yet
- Cbcs SyllabusDocument28 pagesCbcs SyllabusJoydeb BhattacharyyaNo ratings yet
- ME 3131 Syllabus, Format, Grading System1st Sem 2023-2024Document25 pagesME 3131 Syllabus, Format, Grading System1st Sem 2023-2024NICOLE ANN MARCELINONo ratings yet
- CHEM 105 Sample Midterm 2 QuestionsDocument10 pagesCHEM 105 Sample Midterm 2 QuestionsxxdanxxfoxNo ratings yet
- 22MATE11Document5 pages22MATE11New GenieNo ratings yet
- JEE Main 2022 Analysis (June/1st Attempt)Document7 pagesJEE Main 2022 Analysis (June/1st Attempt)Resonance EduventuresNo ratings yet
- Interviewresult HSTSB PGT Biology PGT SanskritDocument124 pagesInterviewresult HSTSB PGT Biology PGT Sanskritram vermaNo ratings yet
- Datesheet SAT Nov. 2022Document1 pageDatesheet SAT Nov. 2022ram vermaNo ratings yet
- NVS TGT North East Region Result Haryana JobsDocument19 pagesNVS TGT North East Region Result Haryana Jobsram vermaNo ratings yet
- ICHR Recruitment 2023 NotificationDocument3 pagesICHR Recruitment 2023 Notificationram vermaNo ratings yet
- KVS Exam Date Notice 2023 HaryanaJobsDocument1 pageKVS Exam Date Notice 2023 HaryanaJobsram vermaNo ratings yet
- 12th Chemistry Syllabus 2022-23Document5 pages12th Chemistry Syllabus 2022-23ram vermaNo ratings yet
- Shoe IndustryDocument10 pagesShoe Industryakusmarty100% (1)
- Succes Pattern SDN BHD ProfileDocument52 pagesSucces Pattern SDN BHD ProfileNadym Gulam RasulNo ratings yet
- Bowen Bowen University: OyepaDocument2 pagesBowen Bowen University: OyepaLO NI MINo ratings yet
- 2002 Issue 1 - What's So Controversial About The New Controversy? - Counsel of ChalcedonDocument4 pages2002 Issue 1 - What's So Controversial About The New Controversy? - Counsel of ChalcedonChalcedon Presbyterian ChurchNo ratings yet
- TSB Gen 049Document3 pagesTSB Gen 049Trọng Nghĩa VõNo ratings yet
- Cheb Yshev PolynomialsDocument13 pagesCheb Yshev PolynomialsBolitten VianeyNo ratings yet
- Endocrine Glands 1 PDFDocument10 pagesEndocrine Glands 1 PDFBalakrishnan MarappanNo ratings yet
- Find The Perfect Logo For Mobilni - SvetDocument1 pageFind The Perfect Logo For Mobilni - SvetRelja IlicNo ratings yet
- Module 3 - Philippine LiteratureDocument9 pagesModule 3 - Philippine LiteratureLyn PalmianoNo ratings yet
- Math Fact Worksheets - Free Printable Math PDFsDocument12 pagesMath Fact Worksheets - Free Printable Math PDFsJordan ShaneNo ratings yet
- Reproductive Health Udaan Dpp1Document14 pagesReproductive Health Udaan Dpp1noobNo ratings yet
- DLL G6 Tleia Q3 W8Document7 pagesDLL G6 Tleia Q3 W8Jose Pasag ValenciaNo ratings yet
- H14YS-5B-2309-Reminder - 011424Document4 pagesH14YS-5B-2309-Reminder - 011424BẢO Nhi LêNo ratings yet
- FT 90rDocument64 pagesFT 90rGuinness88DrinkerNo ratings yet
- Syllabus: Summary of Information On Each CourseDocument10 pagesSyllabus: Summary of Information On Each CourseASMANo ratings yet
- Essay English 20th Anniversary of Oasis Debut Album.Document3 pagesEssay English 20th Anniversary of Oasis Debut Album.Luis Manuel Cárcamo MoralesNo ratings yet
- AO1 What Is Rock ClimbingDocument10 pagesAO1 What Is Rock ClimbingSBNoadNo ratings yet
- Me470l Project Evaluation MechanicalDocument10 pagesMe470l Project Evaluation MechanicalGouravNo ratings yet
- Essay On Crusade EvangelismDocument5 pagesEssay On Crusade EvangelismShakielGibbonsNo ratings yet
- José Rizal Family Tree - Philippine Folklife Museum Foundation - San Francisco, CaDocument3 pagesJosé Rizal Family Tree - Philippine Folklife Museum Foundation - San Francisco, CaBobby QuintosNo ratings yet
- Architectural CompetitionsDocument15 pagesArchitectural CompetitionsShailendra PrasadNo ratings yet
- Solved Marcy Tucker Received The Following Items This Year Determine ToDocument1 pageSolved Marcy Tucker Received The Following Items This Year Determine ToAnbu jaromiaNo ratings yet
- 367 ErpBookDocument13 pages367 ErpBooksravankumar248No ratings yet