Professional Documents
Culture Documents
Ionic Compounds
Uploaded by
Rammohan Balaji Prasad0 ratings0% found this document useful (0 votes)
10 views1 pageIonic compounds are crystalline solids with high melting and boiling points that are soluble in water but not organic solvents. They conduct electricity when molten or dissolved in water due to the movement of ions, but do not conduct in solid state where ions cannot move. Simple covalent compounds are often liquids or gases with low melting and boiling points, insoluble in water but soluble in organic solvents. They do not conduct electricity in any state.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentIonic compounds are crystalline solids with high melting and boiling points that are soluble in water but not organic solvents. They conduct electricity when molten or dissolved in water due to the movement of ions, but do not conduct in solid state where ions cannot move. Simple covalent compounds are often liquids or gases with low melting and boiling points, insoluble in water but soluble in organic solvents. They do not conduct electricity in any state.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
10 views1 pageIonic Compounds
Uploaded by
Rammohan Balaji PrasadIonic compounds are crystalline solids with high melting and boiling points that are soluble in water but not organic solvents. They conduct electricity when molten or dissolved in water due to the movement of ions, but do not conduct in solid state where ions cannot move. Simple covalent compounds are often liquids or gases with low melting and boiling points, insoluble in water but soluble in organic solvents. They do not conduct electricity in any state.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
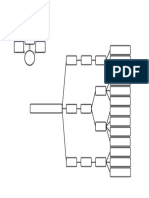
Ionic Compounds Simple Covalent Compounds
Property Reason Property Reason
They are crystalline 1. Ions are arranged in a regular They are often liquid or 1. Simple covalent substances consist of
solids at room arrangement forming a lattice gases at room small molecules.
temperature 2. Ions of the opposite charge are next temperature 2. The forces between the molecules are
to each other, held by strong weak, the molecules are not held together
electrostatic force of attraction. tightly as the particles in a solid, but are free
to move.
They have high 1. Ions are held together by strong They have low melting 1. In simple covalent molecules, the covalent
melting and boiling electrostatic force of attraction. and boiling points bonds between the atoms are strong.
points 2. To melt an ionic compound, a large However the forces between the molecules
amount of heat is needed to break the are weak. These forces are easily broken.
strong attractive forces holding the 2. Note that during melting or boiling, only the
ions together. molecules separate; the atoms in the
molecules do not separate.
They are often 1. The charged ions attract the water They are usually Simple covalent molecules do not have
soluble in water but molecules, which disrupts the lattice insoluble in water but charged ions, so they do not attract water
insoluble in organic structure, causing the ions to separate soluble in organic molecules. Hence they do not dissolve in
solvents and go into the solution. solvents such as petrol. water.
2. The ions do not attract molecules of
organic solvents and so the solid does
not dissolve.
They conduct 1. The ions are free to move in the They do NOT conduct To conduct electricity substances must
electricity when molten state or when dissolved in electricity contain either ions or electrons that are free
molten or when water. The moving ions carry the to move. Simple covalent molecules contain
dissolved in water electric current. neither. So they do not conduct electricity.
but do not conduct 2. In solid state the ions are not free to
electricity in solid move so the current cannot flow.
state.
You might also like
- GCSE Chemistry Revision: Cheeky Revision ShortcutsFrom EverandGCSE Chemistry Revision: Cheeky Revision ShortcutsRating: 4.5 out of 5 stars4.5/5 (3)
- DifferencebetweenionicandcovalentompoundsDocument2 pagesDifferencebetweenionicandcovalentompoundsRammohan Balaji PrasadNo ratings yet
- Chemical Bonding: Understanding The Forces that Hold Molecules Together.From EverandChemical Bonding: Understanding The Forces that Hold Molecules Together.No ratings yet
- Why To Study - Material Technology & Heat TreatmentDocument22 pagesWhy To Study - Material Technology & Heat TreatmentdNo ratings yet
- Practice Makes Perfect in Chemistry: Chemical BondingFrom EverandPractice Makes Perfect in Chemistry: Chemical BondingRating: 5 out of 5 stars5/5 (3)
- Chemical BondingDocument5 pagesChemical BondingSANDEEP SINGHNo ratings yet
- Bonding and StructureDocument20 pagesBonding and StructureYusma KhanNo ratings yet
- Bonding A Level NotesDocument5 pagesBonding A Level NotesWashington NyakaviNo ratings yet
- Crystalline Solids: 4. Clean Cleavage With KnifeDocument5 pagesCrystalline Solids: 4. Clean Cleavage With KnifeShin Se KyungNo ratings yet
- Lesson3 - Chemical Bonding PDFDocument6 pagesLesson3 - Chemical Bonding PDFMia ChanNo ratings yet
- Grade 9 Chemical BondingDocument10 pagesGrade 9 Chemical BondingAmonique DaveyNo ratings yet
- Ionic Molecular Covalent Network Covalent MetallicDocument2 pagesIonic Molecular Covalent Network Covalent MetallicLeah RualesNo ratings yet
- Carbon Is of Immense Significance To Us in Both Its Elemental Form and in The Combined FormDocument2 pagesCarbon Is of Immense Significance To Us in Both Its Elemental Form and in The Combined FormkalloliNo ratings yet
- Ionic CompoundsDocument1 pageIonic CompoundsRobin The peace warriorNo ratings yet
- Forces of AttractionDocument21 pagesForces of AttractionDoveNo ratings yet
- Comparing Covalent and Ionic Lattices S4Document3 pagesComparing Covalent and Ionic Lattices S4Fatima Ahmed-VeriterNo ratings yet
- 4 14 Chemical Bonding 1 Ionic Metallic Bonding JL EditedDocument23 pages4 14 Chemical Bonding 1 Ionic Metallic Bonding JL EditedFN5052023 PRAMITA MAHENDRANNo ratings yet
- Chemical BondingDocument3 pagesChemical BondingKateNo ratings yet
- The Kinetic Molecular Model of Liquids & SolidsDocument2 pagesThe Kinetic Molecular Model of Liquids & SolidschristineNo ratings yet
- Comparing Ionic and Covalent Compounds: RememberDocument1 pageComparing Ionic and Covalent Compounds: RememberAshrafNo ratings yet
- Chemical Bonding NotesDocument5 pagesChemical Bonding NotesShaswat PattnayakNo ratings yet
- GenChem (Lesson 1)Document2 pagesGenChem (Lesson 1)abgolena5238valNo ratings yet
- Bonding in Chemistry NotesDocument19 pagesBonding in Chemistry Notesteacher_555No ratings yet
- Kinetic Molecular ModelDocument3 pagesKinetic Molecular ModelChristine FernandezNo ratings yet
- 2022.08.29 Chemical Components of Cells PT 1 (Ch2)Document35 pages2022.08.29 Chemical Components of Cells PT 1 (Ch2)Allison KwanNo ratings yet
- Properties of Ionic and CovalentDocument1 pageProperties of Ionic and CovalentJoselyn Villena MarquezNo ratings yet
- Design Lab: Investigating Properties of Ionic and Covalent Compounds Using Commonly Used CompoundsDocument2 pagesDesign Lab: Investigating Properties of Ionic and Covalent Compounds Using Commonly Used Compoundsdhairya gandhiNo ratings yet
- Last CJHDocument1 pageLast CJHsoonh jatoiNo ratings yet
- Covalent Bonding, Structure Lecture FileDocument17 pagesCovalent Bonding, Structure Lecture FileMahi QuaziNo ratings yet
- General ChemistryDocument10 pagesGeneral ChemistryKatrine Visitacion Dela CruzNo ratings yet
- Matter: Kinetic EnergyDocument16 pagesMatter: Kinetic EnergyRazel ForrosueloNo ratings yet
- Lesson 3 Chemical PropertiesDocument32 pagesLesson 3 Chemical PropertiesJohann LeoncitoNo ratings yet
- Lecture NotesDocument3 pagesLecture NotesIdk UlitNo ratings yet
- Biochemistry 2nd SemesterDocument46 pagesBiochemistry 2nd SemesterEmelly Galvez PadillaNo ratings yet
- Comparing Ionic & Covalent BondingDocument2 pagesComparing Ionic & Covalent BondingRyan MarvinNo ratings yet
- Intermolecular Forces of Attraction Description 2 Examples: Asynchronous Activity (24 POINTS)Document2 pagesIntermolecular Forces of Attraction Description 2 Examples: Asynchronous Activity (24 POINTS)Joshtine AngoluanNo ratings yet
- General Chemistry 2 1Document16 pagesGeneral Chemistry 2 1blackmage016No ratings yet
- RadioactivityDocument30 pagesRadioactivitybrianna brownNo ratings yet
- General Chemistry 2 Q1 ReviewerDocument10 pagesGeneral Chemistry 2 Q1 ReviewerDuke FaciolNo ratings yet
- Intermolecular and Intramolecular ForcesDocument2 pagesIntermolecular and Intramolecular ForcesDev SinghNo ratings yet
- Bonding and Properties of SubstancesDocument3 pagesBonding and Properties of Substancesdan964No ratings yet
- PS 12 Midterm ReviewerDocument14 pagesPS 12 Midterm ReviewerMaribeth Alyssa GoNo ratings yet
- Notes3 Unit1Document2 pagesNotes3 Unit1arun iyer BitcoinminerandmathematicianNo ratings yet
- Property Explanation: Liquid StateDocument9 pagesProperty Explanation: Liquid StateNothing NameNo ratings yet
- Bonding and Structure: Chemistry Notes GCE Study BuddyDocument17 pagesBonding and Structure: Chemistry Notes GCE Study BuddyKhemou DjvickzNo ratings yet
- Biology NotesDocument60 pagesBiology Noteskiler2314No ratings yet
- Biology 101 NotesDocument60 pagesBiology 101 Noteskiler2314No ratings yet
- Structure and BondingDocument1 pageStructure and BondingeohomegrownappsNo ratings yet
- Ionic Covalent PresentationDocument10 pagesIonic Covalent PresentationDanilo Fronda Jr.No ratings yet
- Ionic/Covalent Bonding NotesDocument10 pagesIonic/Covalent Bonding NotesDanilo Fronda Jr.No ratings yet
- Ionic/Covalent Bonding NotesDocument10 pagesIonic/Covalent Bonding NotesJAGADISH S 1940472No ratings yet
- Bonding Summary NotesDocument17 pagesBonding Summary NotesaleenNo ratings yet
- Inquiry Into Bonding Lab - Intro TestDocument5 pagesInquiry Into Bonding Lab - Intro Testapi-491531529No ratings yet
- Chapter 2: Chemistry Comes Alive (Marieb)Document17 pagesChapter 2: Chemistry Comes Alive (Marieb)Kayte Middleton100% (1)
- Gen Chem 002Document7 pagesGen Chem 002jazz vergsNo ratings yet
- Bonding Knowledge OrganiserDocument1 pageBonding Knowledge Organisermya thet htar sweNo ratings yet
- Properties of CompoundsDocument2 pagesProperties of CompoundsAngel OghayonNo ratings yet
- Gen ChemistryDocument2 pagesGen ChemistryFrench Erica ManlapasNo ratings yet
- Gen Chem w1-2Document6 pagesGen Chem w1-2Cyril FaithNo ratings yet
- Essentials of The Living World 5th Edition George Johnson Solutions ManualDocument4 pagesEssentials of The Living World 5th Edition George Johnson Solutions Manuala779655316No ratings yet
- Science Laboratory Rules - NEWDocument3 pagesScience Laboratory Rules - NEWRammohan Balaji PrasadNo ratings yet
- Lab Equipment Names and PicturDocument8 pagesLab Equipment Names and PicturRammohan Balaji PrasadNo ratings yet
- Common Lab Apparatus - Cut & StickDocument2 pagesCommon Lab Apparatus - Cut & StickRammohan Balaji PrasadNo ratings yet
- Common Lab Apparatus and ProcedureDocument12 pagesCommon Lab Apparatus and ProcedureRammohan Balaji PrasadNo ratings yet
- Chemistry Lab Manual FinalDocument69 pagesChemistry Lab Manual FinalRammohan Balaji PrasadNo ratings yet
- Structure and Bonding SummaryDocument1 pageStructure and Bonding SummaryRammohan Balaji PrasadNo ratings yet
- Notes Chemical BondingDocument16 pagesNotes Chemical BondingRammohan Balaji PrasadNo ratings yet
- Giant Molecular Structure WsDocument5 pagesGiant Molecular Structure WsRammohan Balaji PrasadNo ratings yet
- Bonding Card SortDocument2 pagesBonding Card SortRammohan Balaji PrasadNo ratings yet
- A Comprehensive Review On Textile Waste Valorization Techniques and Their ApplicationsDocument16 pagesA Comprehensive Review On Textile Waste Valorization Techniques and Their ApplicationsDavid WashingtonNo ratings yet
- 2K Solvent Free Waterproofing FormulationDocument2 pages2K Solvent Free Waterproofing FormulationM Idrees100% (2)
- Handbook of Detergents Part C - AnalysisDocument669 pagesHandbook of Detergents Part C - AnalysisPeter100% (6)
- 0620 m15 Ms 22Document6 pages0620 m15 Ms 22sookchinNo ratings yet
- Mechanistic Bioinorganic Chemistry (1995) PDFDocument501 pagesMechanistic Bioinorganic Chemistry (1995) PDFSORIN AVRAMESCUNo ratings yet
- Redox Reactions - Shobhit NirwanDocument12 pagesRedox Reactions - Shobhit NirwanAadarsh PandeyNo ratings yet
- Disperse Printing ProcessDocument32 pagesDisperse Printing Processade rahayuNo ratings yet
- Aeronomy of The Middle Atmosphere - 2005Document651 pagesAeronomy of The Middle Atmosphere - 2005DaviMouraNo ratings yet
- Bio Lab Report (G2) PDFDocument10 pagesBio Lab Report (G2) PDFAina NabihaNo ratings yet
- Chapter 1 Elementary Materials Science PDFDocument33 pagesChapter 1 Elementary Materials Science PDFSohan HasanNo ratings yet
- Chloroplasts and Chlorophyll T5-3Document4 pagesChloroplasts and Chlorophyll T5-3Kyile FernandoNo ratings yet
- Study Material Science 2023-24Document308 pagesStudy Material Science 2023-24Tapas BanerjeeNo ratings yet
- Analysis of Propolis - Some Parameters and Procedures For Chemical Quality ControlDocument8 pagesAnalysis of Propolis - Some Parameters and Procedures For Chemical Quality ControlEmanoelNo ratings yet
- Adsorption ProcessDocument24 pagesAdsorption ProcessMuhammad AlghitanyNo ratings yet
- Patent Application Publication (10) Pub. No.: US 2014/0100293 A1Document17 pagesPatent Application Publication (10) Pub. No.: US 2014/0100293 A1JutonoNo ratings yet
- Titanium Alloys in Total Joint Replacement - A Materials Science PerspectiveDocument9 pagesTitanium Alloys in Total Joint Replacement - A Materials Science PerspectiveSatria Adi NugrohoNo ratings yet
- HYDROCARBONSDocument13 pagesHYDROCARBONSsophia del rosarioNo ratings yet
- 3 Amino AcidsDocument45 pages3 Amino AcidsBereket Agumas KegneNo ratings yet
- BioPolymer Based Packaging BookDocument489 pagesBioPolymer Based Packaging BookPrabhuPalanichamy100% (1)
- Final Edited SPIONs Report PDFDocument22 pagesFinal Edited SPIONs Report PDFshoaib ahmedNo ratings yet
- Worksheet-Haloalkanes and HaloarenesDocument5 pagesWorksheet-Haloalkanes and HaloarenesAslamNo ratings yet
- Bio 1Document7 pagesBio 1Margie Andrea Peña Lopez ESTUDIANTE ACTIVONo ratings yet
- Multiple Choice Questions in Biochemistry: Digital Edition © RC GuptaDocument16 pagesMultiple Choice Questions in Biochemistry: Digital Edition © RC Guptatekalignpawulose09No ratings yet
- Solid Dosage FormsDocument40 pagesSolid Dosage FormsAndy EgutNo ratings yet
- Science Class X Sample Paper Test 03 For Board Exam 2024Document7 pagesScience Class X Sample Paper Test 03 For Board Exam 2024Aayushi Mishra100% (1)
- Bio 150-Experiment 1Document8 pagesBio 150-Experiment 1Aina MaisarahNo ratings yet
- CRE Notes PDFDocument61 pagesCRE Notes PDFKrunal ThakarNo ratings yet
- Hydroformylation - Hydrogenation of Ethylene OxideDocument9 pagesHydroformylation - Hydrogenation of Ethylene OxideSagar BansodNo ratings yet
- Lesson 4 - The Polarity of Molecule Based On Its StructureDocument48 pagesLesson 4 - The Polarity of Molecule Based On Its Structuretheresa balaticoNo ratings yet
- Balanced Equations & Associated Calc's 05 MSDocument7 pagesBalanced Equations & Associated Calc's 05 MSlmao lmaoNo ratings yet