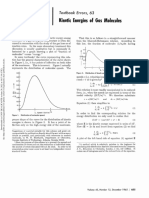
Professional Documents
Culture Documents
Ed080p1258 1
Ed080p1258 1
Uploaded by
Aitor PastorOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ed080p1258 1
Ed080p1258 1
Uploaded by
Aitor PastorCopyright:
Available Formats
Chemical Education Today
Letters
→) vs Equations (⫽)
Reactions (→ Laidler. However, the solution proposed in their report (3)
was that the two should be denoted by slightly different types
A paper by Toby (1) has referred to the important dis- of arrow, one with a simple arrowhead (→) and the other with
tinction between chemical reactions and chemical equations. a filled arrowhead (➝). At best, this method of distinction
In this, Toby states, “Reactions are signified by an arrow would seem (no pun intended) a rather blunt weapon and in
meaning ‘becomes’ … whereas equations are signified by an certain fonts the difference might not be clearly apparent.
equals sign…”, and has credited this distinction to Diemente A recent chemical kinetics text (4) has adopted a differ-
(2). However, neither author seems to have employed this ent solution, using an equals sign for the overall reaction:
mode of distinction in a totally consistent way.
H2 ⫹ Br2 ⫽ 2HBr
In chemistry, we may use chemical equations in various
ways. In some instances we regard it as very important that whereas an arrow, →, is used in each of the reaction steps
our equation denotes the process that actually occurs, and that are proposed to constitute the mechanism, and for any
this would usually apply in kinetic studies. On the other elementary process. This would seem to be in accord with
See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles.
hand, we may sometimes wish to evaluate the standard en- the policy advocated by Toby (1).
thalpy change of a reaction process that could not conceiv-
ably be achieved in the laboratory. So for simplicity, let me Literature Cited
Downloaded via UNIV DE ALICANTE on February 6, 2023 at 21:30:41 (UTC).
confine my remarks to the area of kinetics, which is the one 1. Toby, S. J. Chem. Educ. 2000, 77, 188–190.
to which Toby (1) has referred. 2. Diemente, K. J. Chem. Educ. 1998, 75, 319–321.
Some reactions are known to be elementary processes, 3. Laidler, K. J. Pure and Applied Chem. 1981, 53, 753–771.
occurring in a single step involving precisely those species writ- 4. Logan, S. R. Fundamentals of Chemical Kinetics; Longman:
ten on the left-hand side of the balanced equation, and some Harlow, England, 1996.
are known not to be. For the latter, an important and neces-
sary distinction is that between the overall reaction process S. R. Logan
and the individual reactions steps by which this process is School of Biomedical Sciences, University of Ulster
achieved. This was recognized, for example, by the IUPAC Coleraine, N. Ireland BT52 1SA
Sub-Committee on Chemical Kinetics, chaired by K. J. sr.logan@ulster.ac.uk
1258 Journal of Chemical Education • Vol. 80 No. 11 November 2003 • JChemEd.chem.wisc.edu
You might also like
- 05 0846 02 MS 8RP AFP tcm142-654612Document16 pages05 0846 02 MS 8RP AFP tcm142-654612David Weynom83% (6)
- 8.1 Worksheet (Answer Key)Document5 pages8.1 Worksheet (Answer Key)Black arab GaladimaNo ratings yet
- 3rd Quarter TOS GRADE 9Document1 page3rd Quarter TOS GRADE 9Diosdado II Marimon100% (8)
- DeepLines Training (1) - OverviewDocument94 pagesDeepLines Training (1) - OverviewTran Thanh TungNo ratings yet
- Lesson 3: Chemical Reactions and Equation: EO: DefineDocument6 pagesLesson 3: Chemical Reactions and Equation: EO: DefineEdgardo VILLASEÑORNo ratings yet
- Polymers at Interfaces and The Interactions in Colloidal DispersionsDocument13 pagesPolymers at Interfaces and The Interactions in Colloidal DispersionsImalka PriyadarshaniNo ratings yet
- Minakshee ChEngJ2019Document10 pagesMinakshee ChEngJ2019K Suresh AkkihebbalNo ratings yet
- Daily Lesson Log: Dipaculao NHS 11Document5 pagesDaily Lesson Log: Dipaculao NHS 11Dondon TayabanNo ratings yet
- Hill 1986Document6 pagesHill 1986bryan beleño acostaNo ratings yet
- Stoichiometry of Equations Through The Inverse de Donder RelationDocument6 pagesStoichiometry of Equations Through The Inverse de Donder Relationski3013No ratings yet
- The Notion of Hormesis and The Dose-Response Theory: A Unified ApproachDocument11 pagesThe Notion of Hormesis and The Dose-Response Theory: A Unified ApproachAnonymous wzDPyhNo ratings yet
- Stoichiometry of Equations Through The Inverse deDocument6 pagesStoichiometry of Equations Through The Inverse demuhammaddede23idNo ratings yet
- Sir DollanoDocument10 pagesSir DollanoMANLIGAS, GLENN PAUL L.No ratings yet
- Shimizu, S. (2020) - Formulating Rationally Via Statistical Thermodynamics. Curr. Opin. Colloid Interface Sci. 48, 53-64Document12 pagesShimizu, S. (2020) - Formulating Rationally Via Statistical Thermodynamics. Curr. Opin. Colloid Interface Sci. 48, 53-64marco_ravelo_10No ratings yet
- C5FD90047FDocument34 pagesC5FD90047FHilios MonosNo ratings yet
- J4 BahanDocument13 pagesJ4 Bahanmr mustofaNo ratings yet
- Soal-Soal KD TroDocument24 pagesSoal-Soal KD TroTita Dian NofitaNo ratings yet
- 3.5 Unit 5 CHEM5 Energetics, Redox and Inorganic Chemistry: ThermodynamicsDocument5 pages3.5 Unit 5 CHEM5 Energetics, Redox and Inorganic Chemistry: Thermodynamicsjohn mNo ratings yet
- With Inclusion of The Provisions of Deped Order No. 8, S. 2015Document4 pagesWith Inclusion of The Provisions of Deped Order No. 8, S. 2015Catherine VillaruzNo ratings yet
- Assigning Reactive Excited States in Inorganic PhotochemistryDocument14 pagesAssigning Reactive Excited States in Inorganic PhotochemistryKannamundayil BakesNo ratings yet
- IUPAC Recomendations For MechanismDocument7 pagesIUPAC Recomendations For MechanismRenato Pereira Pinheiro100% (1)
- Definición de Sistema 1Document4 pagesDefinición de Sistema 1Edson Lozano LafforeNo ratings yet
- Jeb141309 Do Enthoterms TPCR PDFDocument9 pagesJeb141309 Do Enthoterms TPCR PDFAlan David Ruiz GarcíaNo ratings yet
- Decherchi 2015Document16 pagesDecherchi 2015Willy Menacho NNo ratings yet
- Geometric Effects of Reduction of Dimensionality in Interfacial Reactions'Document5 pagesGeometric Effects of Reduction of Dimensionality in Interfacial Reactions'Dean AstumianNo ratings yet
- Substituent Effects On Simple Diels-Alder Reactions: Evidence For Possible Explosive Reactions From Quantum Mechanical CalculationsDocument8 pagesSubstituent Effects On Simple Diels-Alder Reactions: Evidence For Possible Explosive Reactions From Quantum Mechanical CalculationsBaban BaidyaNo ratings yet
- 08 Reaction-Diffusion Systems For Algorithmic Composition - ANDREW MARTINDocument8 pages08 Reaction-Diffusion Systems For Algorithmic Composition - ANDREW MARTINrm4sd8bkdyNo ratings yet
- Mahatma Gandhi Institute of Pharmacy, LucknowDocument1 pageMahatma Gandhi Institute of Pharmacy, LucknowMukesh TiwariNo ratings yet
- Xii ChemistryDocument11 pagesXii Chemistryshahilthakur07No ratings yet
- A Detailed Study On Pericyclic Reactions: Raj Kumar Pandey, Dr. Neelima JainDocument3 pagesA Detailed Study On Pericyclic Reactions: Raj Kumar Pandey, Dr. Neelima JainJennifer Carolina Rosales NoriegaNo ratings yet
- Method of Continuous Variations - Applications of Job Plots To The Study of Molecular Associations in Organometallic ChemistryDocument17 pagesMethod of Continuous Variations - Applications of Job Plots To The Study of Molecular Associations in Organometallic ChemistryYosimayrobi Endo NavarroNo ratings yet
- Prigogine 1968Document7 pagesPrigogine 1968frank ndjomatchouaNo ratings yet
- Chemical Reactions and Chemical Equations: General Chemistry 1Document17 pagesChemical Reactions and Chemical Equations: General Chemistry 1Aries MvillNo ratings yet
- Cinética Química y Diseño de ReactoresDocument90 pagesCinética Química y Diseño de Reactoresquiksilver21No ratings yet
- AP Chem Summer Assignment-2018-19Document56 pagesAP Chem Summer Assignment-2018-19Lei PronceNo ratings yet
- The Elusive Chemical PotentialDocument13 pagesThe Elusive Chemical PotentialMohammad BasitNo ratings yet
- Chemical EquilibriumDocument34 pagesChemical EquilibriumLala Rifa0% (1)
- Bioinformatics: Original PaperDocument8 pagesBioinformatics: Original Paperchelster08No ratings yet
- Physical Science 20 - Lesson Plan OldDocument6 pagesPhysical Science 20 - Lesson Plan Oldapi-349567441No ratings yet
- This Content Downloaded From 159.65.238.93 On Fri, 23 Oct 2020 10:32:44 UTCDocument17 pagesThis Content Downloaded From 159.65.238.93 On Fri, 23 Oct 2020 10:32:44 UTCtanatswaNo ratings yet
- Dose and Time Determining, and Other Factors Influencing, ToxicityDocument99 pagesDose and Time Determining, and Other Factors Influencing, Toxicitymecejo7728No ratings yet
- Study Guide Chapter 2: Life Chemistry and Energy: 0. ApplicationDocument19 pagesStudy Guide Chapter 2: Life Chemistry and Energy: 0. ApplicationKelly WayneNo ratings yet
- CW 41Document13 pagesCW 41lgguillen20No ratings yet
- 6.3 TextbookDocument3 pages6.3 TextbookJoshua AdetoroNo ratings yet
- The Michael ReactionDocument4 pagesThe Michael ReactionJennifer Carolina Rosales NoriegaNo ratings yet
- Additivity Rules For The Estimation of Molecular Properties. Thermodynamic PropertiesDocument28 pagesAdditivity Rules For The Estimation of Molecular Properties. Thermodynamic Propertieswesileh981No ratings yet
- Irreversibility: ThermodynamicDocument4 pagesIrreversibility: ThermodynamicAitor PastorNo ratings yet
- Two Multic ComponetDocument20 pagesTwo Multic ComponetFriday Niyehmi Abolorunke ChristopherNo ratings yet
- 1 Thermolecture 1Document50 pages1 Thermolecture 1extreme fattypunchNo ratings yet
- 1 s2.0 S0010218019301361 MainDocument17 pages1 s2.0 S0010218019301361 Mainedo hangukoNo ratings yet
- Engqvist ANCOVA Interaction TermDocument5 pagesEngqvist ANCOVA Interaction TermNazia SyedNo ratings yet
- Artículo - El Avance de La Reacción IIDocument3 pagesArtículo - El Avance de La Reacción IIEyvind Andres Rondon RinconNo ratings yet
- SCIENCE (52) Chemistry SCIENCE Paper - 2: Class IxDocument11 pagesSCIENCE (52) Chemistry SCIENCE Paper - 2: Class IxDEBASIS SAHOONo ratings yet
- Insights Into The Chemical Meanings of The Reaction Electronic FluxDocument7 pagesInsights Into The Chemical Meanings of The Reaction Electronic FluxDesmonius Lab GroupNo ratings yet
- CHE 254 - Lesson 2 - Thermodynamic Properties of Fluid Mixtures - IDocument17 pagesCHE 254 - Lesson 2 - Thermodynamic Properties of Fluid Mixtures - IEbenezer EffisahNo ratings yet
- SCIENCE (52) Chemistry SCIENCE Paper - 2: Class IxDocument11 pagesSCIENCE (52) Chemistry SCIENCE Paper - 2: Class IxSatyamevNo ratings yet
- Ing SuperficiesDocument10 pagesIng SuperficiesJosé ReyesNo ratings yet
- Claudia Maienborn, Semantics, 201Document35 pagesClaudia Maienborn, Semantics, 201robert guimaraesNo ratings yet
- Olmos2001 2Document7 pagesOlmos2001 2hamza aouaichiaNo ratings yet
- Free Radical Halogenation JCE 2004Document4 pagesFree Radical Halogenation JCE 2004RobertNo ratings yet
- Ch.2.3 WSDocument3 pagesCh.2.3 WS무루파털뭉치No ratings yet
- Reaction Kinetics: Reactions in SolutionFrom EverandReaction Kinetics: Reactions in SolutionRating: 3.5 out of 5 stars3.5/5 (4)
- Ed066p853 2Document1 pageEd066p853 2Aitor PastorNo ratings yet
- 6 CHM 5710 Using Character TablesDocument35 pages6 CHM 5710 Using Character TablesAitor PastorNo ratings yet
- Low-Noise Simplex Optimization Experiment: FutilityDocument2 pagesLow-Noise Simplex Optimization Experiment: FutilityAitor PastorNo ratings yet
- Instrumental: Simplex OptimizationDocument4 pagesInstrumental: Simplex OptimizationAitor PastorNo ratings yet
- For Conceptualization The Franck-Condon PrincipleDocument1 pageFor Conceptualization The Franck-Condon PrincipleAitor PastorNo ratings yet
- Numerical Evaluation of Energy Levels and Wave Functions For Hindered Internal RotationDocument8 pagesNumerical Evaluation of Energy Levels and Wave Functions For Hindered Internal RotationAitor PastorNo ratings yet
- Coordination: Complexes CobaltDocument3 pagesCoordination: Complexes CobaltAitor PastorNo ratings yet
- Spectroscopy,: Laser-Induced Fluorescence Dynamics, DiagnosticsDocument10 pagesSpectroscopy,: Laser-Induced Fluorescence Dynamics, DiagnosticsAitor PastorNo ratings yet
- Of Chemistry: Calculation Factors UndergraduateDocument3 pagesOf Chemistry: Calculation Factors UndergraduateAitor PastorNo ratings yet
- Active: of Optically ComplexDocument2 pagesActive: of Optically ComplexAitor PastorNo ratings yet
- Franck-Condon Factors and Their Use in Undergraduate Quantum MechanicsDocument7 pagesFranck-Condon Factors and Their Use in Undergraduate Quantum MechanicsAitor PastorNo ratings yet
- Air-Sensitive: Argon Techniques Manipulation ofDocument1 pageAir-Sensitive: Argon Techniques Manipulation ofAitor PastorNo ratings yet
- Electronic Tetrahedral Complexes: Nickel (LL)Document2 pagesElectronic Tetrahedral Complexes: Nickel (LL)Aitor PastorNo ratings yet
- Acs Jchemed 5b00170Document5 pagesAcs Jchemed 5b00170Aitor PastorNo ratings yet
- Lattice Prediction Kapustinskii Equations and Born-Haber: Energy and Chemical The TheDocument7 pagesLattice Prediction Kapustinskii Equations and Born-Haber: Energy and Chemical The TheAitor PastorNo ratings yet
- Claisen Rearrangement Over The Past Nine Decades: Ana M. Martı N CastroDocument64 pagesClaisen Rearrangement Over The Past Nine Decades: Ana M. Martı N CastroAitor PastorNo ratings yet
- Textbook Errors, 63 Kinetic Molecules: EnergiesDocument2 pagesTextbook Errors, 63 Kinetic Molecules: EnergiesAitor PastorNo ratings yet
- Ab 036Document6 pagesAb 036Aitor PastorNo ratings yet
- Textbook Errors, 62 Difference Between and Liquids and SolidsDocument2 pagesTextbook Errors, 62 Difference Between and Liquids and SolidsAitor PastorNo ratings yet
- Ed5b00170 Si 001Document27 pagesEd5b00170 Si 001Aitor PastorNo ratings yet
- Error Titrations Mixtures: MinimumDocument4 pagesError Titrations Mixtures: MinimumAitor PastorNo ratings yet
- TheBaldwinRules RevisedandExtendedDocument29 pagesTheBaldwinRules RevisedandExtendedAitor PastorNo ratings yet
- Acssuschemeng 2c00095Document10 pagesAcssuschemeng 2c00095Aitor PastorNo ratings yet
- Microscale Preparation of Alcl3 Journal of ChemicaDocument2 pagesMicroscale Preparation of Alcl3 Journal of ChemicaAitor PastorNo ratings yet
- Styer 2000Document7 pagesStyer 2000Aitor PastorNo ratings yet
- Kinetics: Electrode ProcessesDocument8 pagesKinetics: Electrode ProcessesAitor PastorNo ratings yet
- Acs Joc 8b00707Document14 pagesAcs Joc 8b00707Aitor PastorNo ratings yet
- The Multistep: Is Rate-Limiting ofDocument5 pagesThe Multistep: Is Rate-Limiting ofAitor PastorNo ratings yet
- Appendix A - Conversion From Molar To Molal: PL PLDocument57 pagesAppendix A - Conversion From Molar To Molal: PL PLAitor PastorNo ratings yet
- Textbook: ForumDocument5 pagesTextbook: ForumAitor PastorNo ratings yet
- Angle TheoremsDocument13 pagesAngle TheoremsEdmund Serna100% (1)
- 05-Proses Produksi (Dasar Proses Manufaktur) ARIODocument22 pages05-Proses Produksi (Dasar Proses Manufaktur) ARIOAlifNo ratings yet
- Real Life Graphs Answers MMEDocument2 pagesReal Life Graphs Answers MMECCSC124-Soham MaityNo ratings yet
- (Fifth Revision) : Amendment No. 2 April 2022 TO Is 4984: 2016 Polyethylene Pipes For Water Supply - SpecificationDocument7 pages(Fifth Revision) : Amendment No. 2 April 2022 TO Is 4984: 2016 Polyethylene Pipes For Water Supply - Specificationnarendar.183% (6)
- Cryogenics: Seungwhan Baek, Cheonkyu Lee, Sangkwon JeongDocument13 pagesCryogenics: Seungwhan Baek, Cheonkyu Lee, Sangkwon JeongDedi AfandiNo ratings yet
- Annual Report English 21 22Document272 pagesAnnual Report English 21 22raj kishanNo ratings yet
- AS 1418.2 Cranes, Hoists and Winches Part 2 Serial Hoists and WinchesDocument31 pagesAS 1418.2 Cranes, Hoists and Winches Part 2 Serial Hoists and WinchesDuy PhướcNo ratings yet
- The Wild T1 TheodoliteDocument61 pagesThe Wild T1 TheodoliteCJLara100% (1)
- Diss Module #1Document7 pagesDiss Module #1Jhener NonesaNo ratings yet
- The Significance of The Poynting VectorDocument9 pagesThe Significance of The Poynting VectorFrederick David TombeNo ratings yet
- Duality Estimation Control GoodwinDocument10 pagesDuality Estimation Control GoodwinArthur CostaNo ratings yet
- Engineering MetrologyDocument6 pagesEngineering Metrologysolomon guadeNo ratings yet
- MECHANICAL - Ques & Answ: Srikalahasthi Pipes LTD, Dept.Document2 pagesMECHANICAL - Ques & Answ: Srikalahasthi Pipes LTD, Dept.SANJEET KUMARNo ratings yet
- Module 1. Assessment Answer Each Item. 1. Provide A Concise Answer To The Following Questions: A. What Is Mathematics?Document2 pagesModule 1. Assessment Answer Each Item. 1. Provide A Concise Answer To The Following Questions: A. What Is Mathematics?마이No ratings yet
- TOS in PRE-CALCULUSDocument2 pagesTOS in PRE-CALCULUSSerjohnRapsingNo ratings yet
- Year 9 - Weekly Test 2 - Feb 2021Document7 pagesYear 9 - Weekly Test 2 - Feb 2021Haseeb KhâñNo ratings yet
- Fea Mr71-2018 Part 2 of 2 Parts - Final SubmissionDocument44 pagesFea Mr71-2018 Part 2 of 2 Parts - Final SubmissionRonish ChandraNo ratings yet
- Exertus Circa Operation Manual MKT - OM - CIRCA (Rev.01) - enDocument32 pagesExertus Circa Operation Manual MKT - OM - CIRCA (Rev.01) - enmuni fatuzzahrohNo ratings yet
- Chapter 4 NewDocument36 pagesChapter 4 NewNur ShazwaniNo ratings yet
- 10.1 Material Properties, Development, and Splice LengthsDocument12 pages10.1 Material Properties, Development, and Splice LengthsjoshuaNo ratings yet
- Magnetic Cement MC40: Material DatasheetDocument4 pagesMagnetic Cement MC40: Material DatasheetCristopher AvejaresNo ratings yet
- Experimental Investigation of Machining Parameters For Aluminum 6061 T6 AlloyDocument6 pagesExperimental Investigation of Machining Parameters For Aluminum 6061 T6 AlloyEditor IJTSRDNo ratings yet
- Chapter 10 Simple Machines Study Guide KeyDocument6 pagesChapter 10 Simple Machines Study Guide KeyOlalekan OyekunleNo ratings yet
- The Scientific Revolution in The Middle EastDocument36 pagesThe Scientific Revolution in The Middle EastJade Ocampo100% (1)
- Tosp P038 Exe Dec 6335 Pip RPT 0001Document19 pagesTosp P038 Exe Dec 6335 Pip RPT 0001RamKS80No ratings yet
- Soil ClassificationDocument18 pagesSoil ClassificationKrizsel ContrerasNo ratings yet
- RHP BearingsDocument24 pagesRHP BearingsdeshpravinNo ratings yet