Professional Documents
Culture Documents
M1a1 Estrada
Uploaded by
Asia EstradaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
M1a1 Estrada
Uploaded by
Asia EstradaCopyright:
Available Formats
Name: Asia D.
Estrada Date: March 6, 2023
Year & Section: APS2 M1A1 – Biochemistry
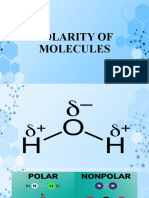
ELECTRONEGATIVITY
1. In a molecule of Carbon Dioxide (CO2),
• What is the electronegativity of carbon?
Answer: 2.55
• What is the electronegativity of oxygen?
Answer: 3.44
• What is the difference in EN of CO2?
Answer: 0.89
• Which atom will have a slightly positive charge?
Answer: Carbon
• Which atom will have a slightly negative charge in the molecule?
Answer: Oxygen
• Is the bond a non-polar or polar covalent bond?
Answer: Non-polar
• Is the molecule polar or non-polar?
Answer: Non-polar
2. Complete the table below:
Molecule Difference in Non-polar/polar
electronegativity between covalent/ionic bond
atoms
H2O 1.5 POLAR COVALENT
HBr 0.76 POLAR COVALENT
F2 0 NON-POLAR MOLECULE
CCl4 0.61 POLAR MOLECULE
MgO 2.13 POLAR MOLECULE
You might also like
- Physical Science Modules Week 2Document6 pagesPhysical Science Modules Week 2RODJHEN ANNE P. BARQUILLANo ratings yet
- Science 9 Q2-Wk 2 - SLHT-2 OkDocument6 pagesScience 9 Q2-Wk 2 - SLHT-2 OkNylana Cañedo del Castillo100% (1)
- Polarity & Electronegativity Worksheet SOLVEDDocument1 pagePolarity & Electronegativity Worksheet SOLVEDLili0% (1)
- PHYSICAL SCIENCE Q1 W2 Mod2 PDFDocument14 pagesPHYSICAL SCIENCE Q1 W2 Mod2 PDFLovely IñigoNo ratings yet
- Week 2 Polarity of Molecules and Its PropertiesDocument38 pagesWeek 2 Polarity of Molecules and Its Propertieslily smithNo ratings yet
- Chemistry Unit 2 Study Guide AnswersDocument6 pagesChemistry Unit 2 Study Guide AnswersH.sNo ratings yet
- Electronegativitiy Worksheet - WELCHDocument5 pagesElectronegativitiy Worksheet - WELCHkamrynwelch1No ratings yet
- Predict Polarity With Electronegativity DiffDocument2 pagesPredict Polarity With Electronegativity DiffRaymond LiuNo ratings yet
- Group 3 Polarity of MoleculeDocument27 pagesGroup 3 Polarity of MoleculeLEA MAXINE MANAIZNo ratings yet
- Q3 Module 3 Polar or Nonpolar: Prepared By: Engr. Erwin D. Rubio JRDocument25 pagesQ3 Module 3 Polar or Nonpolar: Prepared By: Engr. Erwin D. Rubio JRGumban Aaron Frances M.No ratings yet
- Second Midterm ReviewDocument90 pagesSecond Midterm ReviewEvelyn Montserrat Gómez ZentenoNo ratings yet
- Kelly Nolan - Electronegativityworksheet1Document2 pagesKelly Nolan - Electronegativityworksheet1Kelly NolanNo ratings yet
- Organic Chemistry One: Bonding and StructureDocument45 pagesOrganic Chemistry One: Bonding and StructureДууяа Б.No ratings yet
- Chemical Bond (ContDocument18 pagesChemical Bond (ContJachinta JuliusNo ratings yet
- Physical Science Week 4: Name: Rico R. Candelario Grade & Section: 12 St. Gabriel HUMSSDocument4 pagesPhysical Science Week 4: Name: Rico R. Candelario Grade & Section: 12 St. Gabriel HUMSSRico R. CandelarioNo ratings yet
- 2.04-2.05 Intermediate Bonding and Intermolecular Forces PDFDocument16 pages2.04-2.05 Intermediate Bonding and Intermolecular Forces PDFBryan YeohNo ratings yet
- Covalent Bonding Part 1Document32 pagesCovalent Bonding Part 1TaniNo ratings yet
- Polar Bonds&Molecules V2Document9 pagesPolar Bonds&Molecules V2vlattaetaeNo ratings yet
- Properties of Covalent BondingDocument9 pagesProperties of Covalent BondingMBOTAKE LawsonNo ratings yet
- Lecture SlidesDocument32 pagesLecture Slidesabdulqader.nizarNo ratings yet
- Chapter 3Document4 pagesChapter 3叶震森No ratings yet
- C - O Bond Is Polar Since Oxygen Is More Electronegative Than CarbonDocument7 pagesC - O Bond Is Polar Since Oxygen Is More Electronegative Than CarbonYashmi11No ratings yet
- Science ExperimentDocument1 pageScience ExperimentzeravlagwenmarieNo ratings yet
- Physical Science Week 5Document3 pagesPhysical Science Week 5Aira EvangelistaNo ratings yet
- Reveiw - CH06 Bonding KEYDocument5 pagesReveiw - CH06 Bonding KEYMae Seihdrean Bautistä - MAEd Sci 1No ratings yet
- PHYSCI Lesson 5Document33 pagesPHYSCI Lesson 5Paul andrei CasintoNo ratings yet
- Math ReviewDocument3 pagesMath ReviewmasamocmarquishaNo ratings yet
- Midterm Review 2Document82 pagesMidterm Review 2middletown njNo ratings yet
- Unit 4 Electrochemical EnergyDocument49 pagesUnit 4 Electrochemical EnergyRitchel Conde BoholNo ratings yet
- Molecular Orbital TheoryDocument58 pagesMolecular Orbital Theoryvatsala soniNo ratings yet
- 4.2 Electronegativity KEY 2glvvqzDocument2 pages4.2 Electronegativity KEY 2glvvqzAbrogena, Daniela Adiel A.No ratings yet
- 5.types of Covalent CompoundsDocument17 pages5.types of Covalent CompoundsEian InganNo ratings yet
- 12 Jan Eng VWF and MotDocument15 pages12 Jan Eng VWF and Motsachin anuseNo ratings yet
- 03 Electrochemistry Study Guide - Multiple ChoiceDocument22 pages03 Electrochemistry Study Guide - Multiple ChoiceGopal Penjarla100% (1)
- Chemistry (MAke It Easy To Learn)Document4 pagesChemistry (MAke It Easy To Learn)Ashraf ShaharudinNo ratings yet
- Polar and Non PolarDocument22 pagesPolar and Non PolarRowena FloresNo ratings yet
- UGSemsterSyllabus Chemistry 6Sem614Chemistry English InorganicOrganicPhysicalChemistryDocument168 pagesUGSemsterSyllabus Chemistry 6Sem614Chemistry English InorganicOrganicPhysicalChemistryAnil GugulothNo ratings yet
- Chm131 Chapter 3 Chemical Bonds1Document68 pagesChm131 Chapter 3 Chemical Bonds1Adibah Qistina QistinaNo ratings yet
- Mot & Redox ReactionDocument60 pagesMot & Redox ReactionALEENANo ratings yet
- Physical Science Smile 2Document12 pagesPhysical Science Smile 2DYLANNo ratings yet
- CHE101 ChemicalBondingII FZDDocument203 pagesCHE101 ChemicalBondingII FZDsouadalkabirNo ratings yet
- Chemical BondingDocument218 pagesChemical BondingveronicamniemNo ratings yet
- Ionic and CovalentDocument21 pagesIonic and CovalentRobeth EspanoNo ratings yet
- Structure and Reactivity of Organic MoleculeDocument36 pagesStructure and Reactivity of Organic MoleculeAppleNo ratings yet
- 1.7.5 Covalent Bond RevisionDocument3 pages1.7.5 Covalent Bond RevisionTomáš Tommy NagyNo ratings yet
- Sci.9 Weeks 3-4Document4 pagesSci.9 Weeks 3-4Jaime CrispinoNo ratings yet
- Chemistry Final Exam Review KEY: Practice ProblemsDocument7 pagesChemistry Final Exam Review KEY: Practice ProblemsZetrix JensenNo ratings yet
- Chapter 6 CompoundsDocument49 pagesChapter 6 CompoundsUrooj GulNo ratings yet
- 5.1 The Periodic Table: Chemical Periodicity: Atomic RadiusDocument7 pages5.1 The Periodic Table: Chemical Periodicity: Atomic RadiusPedro Moreno de SouzaNo ratings yet
- CombinepdfDocument25 pagesCombinepdfbejeweled1308No ratings yet
- SCH 206 Week 1 Lecture NotesDocument52 pagesSCH 206 Week 1 Lecture NotesDemis ZelelewNo ratings yet
- Polar MoleculesDocument18 pagesPolar MoleculesAly ReyesNo ratings yet
- Science Reviewer by Aneezah PascualDocument5 pagesScience Reviewer by Aneezah Pascualdalialia136iNo ratings yet
- ElectrochemistryModule 4Document6 pagesElectrochemistryModule 4Anushka SinghNo ratings yet
- Organic Chemistry Chapter 2 63-104Document42 pagesOrganic Chemistry Chapter 2 63-104mortemsondeathNo ratings yet
- Covalent Dative Covalent BondingDocument52 pagesCovalent Dative Covalent BondingDearbhla HubbardNo ratings yet
- Polarity of MoleculesDocument25 pagesPolarity of MoleculesAnalynAsuncionAtaydeNo ratings yet
- 1.7. Types of ReactionsDocument10 pages1.7. Types of ReactionsRXNOFCHMNo ratings yet
- Polarity in Covalent BondsDocument15 pagesPolarity in Covalent BondsMarcoNo ratings yet
- Electronic Charges of Bonds in Organic CompoundsFrom EverandElectronic Charges of Bonds in Organic CompoundsRating: 5 out of 5 stars5/5 (1)
- Estrada - Moral Compass PDFDocument2 pagesEstrada - Moral Compass PDFAsia EstradaNo ratings yet
- Reviewer - Mood DisordersDocument10 pagesReviewer - Mood DisordersAsia EstradaNo ratings yet
- Reflection 3 PDFDocument11 pagesReflection 3 PDFAsia EstradaNo ratings yet
- Estrada - Assignment 1 PDFDocument4 pagesEstrada - Assignment 1 PDFAsia EstradaNo ratings yet
- Estrada - Asia Aps2 - Activity 1 PDFDocument4 pagesEstrada - Asia Aps2 - Activity 1 PDFAsia EstradaNo ratings yet