Professional Documents
Culture Documents
GCP Certificate - Prof. Iliya Lozev
Uploaded by
DSpirova0 ratings0% found this document useful (0 votes)
35 views1 pageGCP certificate
Original Title
GCP Certificate_prof. Iliya Lozev
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentGCP certificate
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
35 views1 pageGCP Certificate - Prof. Iliya Lozev
Uploaded by
DSpirovaGCP certificate
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
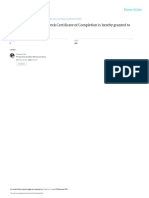
NIDA Clinical Trials Network
Certificate of Completion
is hereby granted to
Iliya Lozev
to certify your completion of the six-hour required course on:
GOOD CLINICAL PRACTICE
MODULE: STATUS:
Introduction N/A
Institutional Review Boards Passed
Informed Consent Passed
Confidentiality & Privacy Passed
Participant Safety & Adverse Events Passed
Quality Assurance Passed
The Research Protocol Passed
Documentation & Record-Keeping Passed
Research Misconduct Passed
Roles & Responsibilities Passed
Recruitment & Retention Passed
Investigational New Drugs Passed
Course Completion Date: 9 May 2023
CTN Expiration Date: 9 May 2026
Eve Jelstrom, Principal Investigator
NDAT CTN Clinical Coordinating Center
Good Clinical Practice, Version 5, effective 03-Mar-2017
This training has been funded in whole or in part with Federal funds from the National Institute on Drug
Abuse, National Institutes of Health, Department of Health and Human Services, under Contract No.
HHSN27201201000024C.
You might also like
- Five Content Areas and Percent of Scored Test Items (Range) in Each AreaDocument5 pagesFive Content Areas and Percent of Scored Test Items (Range) in Each Areaসোমনাথ মহাপাত্রNo ratings yet
- Role of SponsorDocument30 pagesRole of SponsorYellow GutierrezNo ratings yet
- OHST Complete GuideDocument24 pagesOHST Complete GuideFuzail Ayaz100% (1)
- Clinical Research Organization / Contract Research OrganizationDocument34 pagesClinical Research Organization / Contract Research OrganizationPHARMAIGNITENo ratings yet
- Ethical Issues in Clinical TrialsDocument16 pagesEthical Issues in Clinical TrialsPranjal KothaleNo ratings yet
- HLTWHS003 Cluster 7 Changqing GuanDocument38 pagesHLTWHS003 Cluster 7 Changqing GuanSujitha ReddyNo ratings yet
- CSP Complete Guide PDFDocument32 pagesCSP Complete Guide PDFiqjreynaNo ratings yet
- AuditingDocument31 pagesAuditingadamcouples100% (2)
- NIDA Clinical Trials Network: Rene Vanegas PoloDocument1 pageNIDA Clinical Trials Network: Rene Vanegas Polorene vanegas poloNo ratings yet
- Clause 4.2 Interested Parties ListDocument3 pagesClause 4.2 Interested Parties Listwater labNo ratings yet
- GCP Certificate 2017Document1 pageGCP Certificate 2017Mariana0% (1)
- CBIC 2020 Candidate Handbook CBIC 2020 Candidate HandbookDocument16 pagesCBIC 2020 Candidate Handbook CBIC 2020 Candidate HandbookAsif Iqbal100% (1)
- NIDA Clinical Trials Network: Alton DsilvaDocument1 pageNIDA Clinical Trials Network: Alton DsilvaCRCE 9192 AltonNo ratings yet
- Certification HandbookDocument28 pagesCertification HandbookRahul VashishtNo ratings yet
- ISQua SurveyDocument14 pagesISQua SurveySuprapto DR, SPd,SKp,MMNo ratings yet
- Introduction To Information Security Audits and Assessments - SparkDocument12 pagesIntroduction To Information Security Audits and Assessments - SparkCarlNo ratings yet
- Good Clinical PracticeDocument2 pagesGood Clinical PracticeNicholeGarcesCisneros0% (3)
- NIDA CertDocument1 pageNIDA CertMargarita Limon BalunesNo ratings yet
- NIDA Clinical Trial NetworkDocument1 pageNIDA Clinical Trial NetworkNitesh NishantNo ratings yet
- CertificateDocument1 pageCertificatembibaellen8No ratings yet
- Certificate DR DayatDocument1 pageCertificate DR DayatIndraSeptianNo ratings yet
- Certificado Todo PDFDocument12 pagesCertificado Todo PDFLiss VillacresesNo ratings yet
- Ethics CertificateDocument2 pagesEthics Certificateprajyotmagadum3304No ratings yet
- NIDA Clinical Trials Network: Niray MontoyaDocument1 pageNIDA Clinical Trials Network: Niray MontoyaJuan David MartinNo ratings yet
- Inv Learner Guide v2.1Document10 pagesInv Learner Guide v2.1RamyNo ratings yet
- Way To Quality: Accreditation Process: Dr. B.K. RanaDocument36 pagesWay To Quality: Accreditation Process: Dr. B.K. RanaThilinaAbhayarathneNo ratings yet
- Outcomes of Ebp Process 2017Document3 pagesOutcomes of Ebp Process 2017api-272725467100% (1)
- Desarrollo de Base de Evidencia en Acreditación SaludDocument4 pagesDesarrollo de Base de Evidencia en Acreditación SaludNéstor Correa PreciadoNo ratings yet
- Form - LNRDocument6 pagesForm - LNRrobin.rjmNo ratings yet
- Sample Presentation - Science InternshipDocument29 pagesSample Presentation - Science InternshipAmaayaNo ratings yet
- The Team, The Procedures, The Monitor and The Sponsor: Lucy H H Parker Clinical Research Governance ManagerDocument20 pagesThe Team, The Procedures, The Monitor and The Sponsor: Lucy H H Parker Clinical Research Governance ManagerMohammed HammedNo ratings yet
- Quality Assurance in Qualitative ResearchDocument13 pagesQuality Assurance in Qualitative ResearchAndrew TandohNo ratings yet
- Performing The Engagement (Audit Evidence) : John Paolo T. JosonDocument12 pagesPerforming The Engagement (Audit Evidence) : John Paolo T. JosonJohn Paolo JosonNo ratings yet
- PML Company Profile - Clinical TrialDocument11 pagesPML Company Profile - Clinical Trialwisang geniNo ratings yet
- 3655 06 l6 Diploma Qualification HandbookDocument64 pages3655 06 l6 Diploma Qualification Handbooka.mdrashidNo ratings yet
- Validation in Qualitative Research FinalDocument19 pagesValidation in Qualitative Research FinalChama K BenardNo ratings yet
- COMPASSIONATE 3A CLINPATH Activity 1 Comparison of AO 037 and ISO 15189Document17 pagesCOMPASSIONATE 3A CLINPATH Activity 1 Comparison of AO 037 and ISO 15189Adrian CaballesNo ratings yet
- A4 Inv Investigation Courseproviderguide v4 Upload PDFDocument18 pagesA4 Inv Investigation Courseproviderguide v4 Upload PDFjmnringNo ratings yet
- Cqi Pahrio March 27, 2023Document106 pagesCqi Pahrio March 27, 2023KUYA CHICO MORALESNo ratings yet
- كورس الجودةDocument120 pagesكورس الجودةgaber 230No ratings yet
- Study SetupDocument20 pagesStudy SetupROCKER GAMINGNo ratings yet
- Accreditation of Eye Hospitals - A Review: Nirmal Fredrick T, Sunitha NirmalDocument7 pagesAccreditation of Eye Hospitals - A Review: Nirmal Fredrick T, Sunitha Nirmalrgene bioscientificNo ratings yet
- Maiga Ayub Hussein: The African Forum For Research and Education in Health (Afrehealth)Document7 pagesMaiga Ayub Hussein: The African Forum For Research and Education in Health (Afrehealth)Maiga Ayub HusseinNo ratings yet
- ORTHO Clinical DiagnosticsDocument4 pagesORTHO Clinical DiagnosticsHuy Trần ThiệnNo ratings yet
- ControllingDocument8 pagesControllingNonito Patrick GaleraNo ratings yet
- 2016 AAA Study Guide - 16aDocument560 pages2016 AAA Study Guide - 16aGovil KumarNo ratings yet
- J Pak Assoc Dermatol 2012Document5 pagesJ Pak Assoc Dermatol 2012elproedrosNo ratings yet
- Root Cause Analysis and Corrective ActionsTraining - OfficialDocument54 pagesRoot Cause Analysis and Corrective ActionsTraining - OfficialAbdunnajar MahamudNo ratings yet
- CLRI Institute and Course Details Clickable Link Updated July 2022Document32 pagesCLRI Institute and Course Details Clickable Link Updated July 2022shanazk35No ratings yet
- Development of A Guideline - PITIkabi2017Document32 pagesDevelopment of A Guideline - PITIkabi2017Intan Eklesiana NapitupuluNo ratings yet
- 12 Secrets of Effective Test ManagementDocument152 pages12 Secrets of Effective Test ManagementFranco PetruccelliNo ratings yet
- Improving The Validity 621Document110 pagesImproving The Validity 621Ding RarugalNo ratings yet
- Edit Module 2 Fall CRC 2022Document62 pagesEdit Module 2 Fall CRC 2022Raquel VargasNo ratings yet
- Vietnam's First CAP-Accredited Laboratory Boosts Credibility and TrustDocument2 pagesVietnam's First CAP-Accredited Laboratory Boosts Credibility and TrustAmit BiswasNo ratings yet
- Lec-20 Conducting Clinical Trials-Min PDFDocument11 pagesLec-20 Conducting Clinical Trials-Min PDFSatish SinghNo ratings yet
- Module 2.2 - Concept of EBP PDFDocument24 pagesModule 2.2 - Concept of EBP PDFvincyNo ratings yet
- Assignment Investigation (Individu)Document12 pagesAssignment Investigation (Individu)m-6379193No ratings yet
- Arens Aas17 PPT 06Document49 pagesArens Aas17 PPT 06a0939809094No ratings yet
- Iddcr - MB 1 CT ProcessDocument12 pagesIddcr - MB 1 CT ProcessS SreenivasuluNo ratings yet
- Clinical Research Associate - The Comprehensive Guide: Vanguard ProfessionalsFrom EverandClinical Research Associate - The Comprehensive Guide: Vanguard ProfessionalsNo ratings yet