Professional Documents
Culture Documents
NIDA Cert
Uploaded by
Margarita Limon Balunes0 ratings0% found this document useful (0 votes)
64 views1 pageOriginal Title
NIDA cert
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
64 views1 pageNIDA Cert
Uploaded by
Margarita Limon BalunesCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
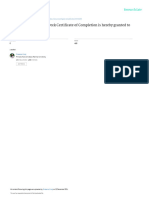
NIDA Clinical Trials Network
Certificate of Completion
is hereby granted to
MARGARITA BALUNES
to certify your completion of the six-hour required course on:
GOOD CLINICAL PRACTICE
MODULE: STATUS:
Introduction N/A
Institutional Review Boards Passed
Informed Consent Passed
Confidentiality & Privacy Passed
Participant Safety & Adverse Events Passed
Quality Assurance Passed
The Research Protocol Passed
Documentation & Record-Keeping Passed
Research Misconduct Passed
Roles & Responsibilities Passed
Recruitment & Retention Passed
Investigational New Drugs Passed
Course Completion Date: 11 March 2022
CTN Expiration Date: 11 March 2025
Andel Frazier, Clinical Training Specialist
NIDA Clinical Coordinating Center
Good Clinical Practice, Version 5, effective 03-Mar-2017
This training has been funded in whole or in part with Federal funds from the National Institute on Drug
Abuse, National Institutes of Health, Department of Health and Human Services, under Contract No.
HHSN27201201000024C.
You might also like
- NIDA Clinical Trials Network: Alton DsilvaDocument1 pageNIDA Clinical Trials Network: Alton DsilvaCRCE 9192 AltonNo ratings yet
- GCP Certificate 2017Document1 pageGCP Certificate 2017Mariana0% (1)
- NIDA Clinical Trials Network: Rene Vanegas PoloDocument1 pageNIDA Clinical Trials Network: Rene Vanegas Polorene vanegas poloNo ratings yet
- GCP Certificate - Prof. Iliya LozevDocument1 pageGCP Certificate - Prof. Iliya LozevDSpirovaNo ratings yet
- NIDA CertificateDocument1 pageNIDA CertificateMyrtle BallesteroNo ratings yet
- Young V RSPCADocument15 pagesYoung V RSPCALiamNo ratings yet
- Imad Ali Shah: Research Methodology, Biostatistics & Dissertation WritingDocument1 pageImad Ali Shah: Research Methodology, Biostatistics & Dissertation Writingsaeedullah afridiNo ratings yet
- Coursera 2LWKFDMH4HJ5Document1 pageCoursera 2LWKFDMH4HJ5Đào Huỳnh PhúcNo ratings yet
- 10th Passing Certificate PDFDocument1 page10th Passing Certificate PDFvishesh bhatiaNo ratings yet
- Coursera SQL CertificateDocument1 pageCoursera SQL Certificateapi-291449456No ratings yet
- CertificateOfCompletion - Statistics Foundations 1Document1 pageCertificateOfCompletion - Statistics Foundations 1Abdul HafeezNo ratings yet
- PAS Learner Completion CertificateDocument3 pagesPAS Learner Completion CertificateAshton Kyle ClarkeNo ratings yet
- Organization Name: ISO 14001:2015 (Environmental Management System)Document1 pageOrganization Name: ISO 14001:2015 (Environmental Management System)RICL Sales ISONo ratings yet
- Fcps Reg CertDocument1 pageFcps Reg CertFARMAN SHAIKH SAHABNo ratings yet
- Final TranscriptDocument4 pagesFinal Transcriptapi-248559244No ratings yet
- Https Covid19.aarogyasri - Telangana.gov - in COVID Covidaction - Do Actionflag generateLabReportOTP&entryId COV4184742 PDFDocument2 pagesHttps Covid19.aarogyasri - Telangana.gov - in COVID Covidaction - Do Actionflag generateLabReportOTP&entryId COV4184742 PDFJayanth GowdaNo ratings yet
- Email - Licence and Statement 1022252Document2 pagesEmail - Licence and Statement 1022252api-397096337No ratings yet
- Health, Safety, and Environment Officer: Ridwan, S.TDocument15 pagesHealth, Safety, and Environment Officer: Ridwan, S.TYusuf NugrohoNo ratings yet
- Cceya CertificateDocument1 pageCceya Certificateapi-702586675No ratings yet
- Emarketing Institute SEO Certification - CERT001224796 EMI PDFDocument1 pageEmarketing Institute SEO Certification - CERT001224796 EMI PDFMaria MEKLINo ratings yet
- Patients Profile: Not Detected NegativeDocument2 pagesPatients Profile: Not Detected NegativeELLIE JAMES PLACIONo ratings yet
- CertificateDocument1 pageCertificatembibaellen8No ratings yet
- Certificate DR DayatDocument1 pageCertificate DR DayatIndraSeptianNo ratings yet
- NIDA Clinical Trial NetworkDocument1 pageNIDA Clinical Trial NetworkNitesh NishantNo ratings yet
- Certificado Todo PDFDocument12 pagesCertificado Todo PDFLiss VillacresesNo ratings yet
- NIDA Clinical Trials Network: Niray MontoyaDocument1 pageNIDA Clinical Trials Network: Niray MontoyaJuan David MartinNo ratings yet
- Ethics CertificateDocument2 pagesEthics Certificateprajyotmagadum3304No ratings yet
- Good Clinical PracticeDocument2 pagesGood Clinical PracticeNicholeGarcesCisneros0% (3)
- Cqi Pahrio March 27, 2023Document106 pagesCqi Pahrio March 27, 2023KUYA CHICO MORALESNo ratings yet
- Way To Quality: Accreditation Process: Dr. B.K. RanaDocument36 pagesWay To Quality: Accreditation Process: Dr. B.K. RanaThilinaAbhayarathneNo ratings yet
- Curriculum Vitae: Quality & Validation Management ProfessionalDocument16 pagesCurriculum Vitae: Quality & Validation Management ProfessionalRamboNo ratings yet
- Role of SponsorDocument30 pagesRole of SponsorYellow GutierrezNo ratings yet
- COMPASSIONATE 3A CLINPATH Activity 1 Comparison of AO 037 and ISO 15189Document17 pagesCOMPASSIONATE 3A CLINPATH Activity 1 Comparison of AO 037 and ISO 15189Adrian CaballesNo ratings yet
- Introduction To Information Security Audits and Assessments - SparkDocument12 pagesIntroduction To Information Security Audits and Assessments - SparkCarlNo ratings yet
- 3655 06 l6 Diploma Qualification HandbookDocument64 pages3655 06 l6 Diploma Qualification Handbooka.mdrashidNo ratings yet
- Accreditation of Eye Hospitals - A Review: Nirmal Fredrick T, Sunitha NirmalDocument7 pagesAccreditation of Eye Hospitals - A Review: Nirmal Fredrick T, Sunitha Nirmalrgene bioscientificNo ratings yet
- Curriculum Vitae: Quality & Validation Management ProfessionalDocument16 pagesCurriculum Vitae: Quality & Validation Management ProfessionalRamboNo ratings yet
- ControllingDocument8 pagesControllingNonito Patrick GaleraNo ratings yet
- OHST Complete GuideDocument24 pagesOHST Complete GuideFuzail Ayaz100% (1)
- Root Cause Analysis and Corrective ActionsTraining - OfficialDocument54 pagesRoot Cause Analysis and Corrective ActionsTraining - OfficialAbdunnajar MahamudNo ratings yet
- CBIC 2020 Candidate Handbook CBIC 2020 Candidate HandbookDocument16 pagesCBIC 2020 Candidate Handbook CBIC 2020 Candidate HandbookAsif Iqbal100% (1)
- The Team, The Procedures, The Monitor and The Sponsor: Lucy H H Parker Clinical Research Governance ManagerDocument20 pagesThe Team, The Procedures, The Monitor and The Sponsor: Lucy H H Parker Clinical Research Governance ManagerMohammed HammedNo ratings yet
- CSP Complete Guide PDFDocument32 pagesCSP Complete Guide PDFiqjreynaNo ratings yet
- NapulanRM - CQI in Records and Clinical Documentation ImprovementDocument92 pagesNapulanRM - CQI in Records and Clinical Documentation ImprovementJeffreyReyesNo ratings yet
- Performing The Engagement (Audit Evidence) : John Paolo T. JosonDocument12 pagesPerforming The Engagement (Audit Evidence) : John Paolo T. JosonJohn Paolo JosonNo ratings yet
- CHST Complete GuideDocument24 pagesCHST Complete GuideFuzail AyazNo ratings yet
- ISQua SurveyDocument14 pagesISQua SurveySuprapto DR, SPd,SKp,MMNo ratings yet
- Study SetupDocument20 pagesStudy SetupROCKER GAMINGNo ratings yet
- Assessor Guide 2016Document42 pagesAssessor Guide 2016Dreana MarshallNo ratings yet
- Sample Presentation - Science InternshipDocument29 pagesSample Presentation - Science InternshipAmaayaNo ratings yet
- Form 1. 2 Evidence of Current Competencies Acquired Related To Job-OccupationDocument2 pagesForm 1. 2 Evidence of Current Competencies Acquired Related To Job-Occupationjayrbayani14No ratings yet
- Your Name: About Me Work ExperienceDocument1 pageYour Name: About Me Work Experiencesaurabh CHATURVEDINo ratings yet
- Outcomes of Ebp Process 2017Document3 pagesOutcomes of Ebp Process 2017api-272725467100% (1)
- Inv Learner Guide v2.1Document10 pagesInv Learner Guide v2.1RamyNo ratings yet
- ORTHO Clinical DiagnosticsDocument4 pagesORTHO Clinical DiagnosticsHuy Trần ThiệnNo ratings yet
- كورس الجودةDocument120 pagesكورس الجودةgaber 230No ratings yet
- AT-01 (Fundamentals of Auditing & Assurance Services)Document5 pagesAT-01 (Fundamentals of Auditing & Assurance Services)Allan MarquezNo ratings yet
- Unit 1 Lecture Slides CFMDocument17 pagesUnit 1 Lecture Slides CFMrealfeelanimeNo ratings yet
- ProQual Level 6 NVQ Diploma in Occupational HS PracticeDocument31 pagesProQual Level 6 NVQ Diploma in Occupational HS PracticeSaddem Hadfi100% (2)
- The Complexity of Process ValidationDocument10 pagesThe Complexity of Process ValidationMiguel Angel Pacahuala CristobalNo ratings yet
- Assisting in Endotracheal SuctioningDocument38 pagesAssisting in Endotracheal SuctioningMargarita Limon BalunesNo ratings yet
- Grapes 1 5Document92 pagesGrapes 1 5Margarita Limon BalunesNo ratings yet
- BibliographyDocument7 pagesBibliographyMargarita Limon BalunesNo ratings yet
- NUR112Document51 pagesNUR112kim torinoNo ratings yet
- P1 DISASTER LEC - LeadershipDocument27 pagesP1 DISASTER LEC - LeadershipDan MichNo ratings yet
- RISHDocument21 pagesRISHMargarita Limon BalunesNo ratings yet
- Grapes TitleDocument6 pagesGrapes TitleMargarita Limon BalunesNo ratings yet
- Sop 3 Tan, ExequielDocument3 pagesSop 3 Tan, ExequielMargarita Limon BalunesNo ratings yet
- Summary of Life and Works of RizalDocument9 pagesSummary of Life and Works of RizalMargarita Limon BalunesNo ratings yet
- Capinding Et Al. Group 1edited DOCSSXDocument88 pagesCapinding Et Al. Group 1edited DOCSSXMargarita Limon BalunesNo ratings yet
- Jsupt Elvis L. Danglose - 4 - 1 - 1Document2 pagesJsupt Elvis L. Danglose - 4 - 1 - 1Margarita Limon BalunesNo ratings yet
- PRESLEYDocument1 pagePRESLEYMargarita Limon BalunesNo ratings yet
- MANSIWORKZ CHAPTER 4 (Done)Document16 pagesMANSIWORKZ CHAPTER 4 (Done)Margarita Limon BalunesNo ratings yet
- CruzDocument1 pageCruzMargarita Limon BalunesNo ratings yet
- Case StudyDocument4 pagesCase StudyMargarita Limon BalunesNo ratings yet
- ConclusionsDocument1 pageConclusionsMargarita Limon BalunesNo ratings yet
- .....Document3 pages.....Margarita Limon BalunesNo ratings yet
- Ubas Approval PENDINGDocument8 pagesUbas Approval PENDINGMargarita Limon BalunesNo ratings yet
- Capinding, Camila O.Document3 pagesCapinding, Camila O.Margarita Limon BalunesNo ratings yet
- CUARESMAJAYFERLSOFTDocument140 pagesCUARESMAJAYFERLSOFTMargarita Limon BalunesNo ratings yet
- Balunes, Margarita L.Document3 pagesBalunes, Margarita L.Margarita Limon BalunesNo ratings yet
- Grapes TitleDocument6 pagesGrapes TitleMargarita Limon BalunesNo ratings yet
- Ballistics ReviewerDocument15 pagesBallistics ReviewerMargarita Limon BalunesNo ratings yet
- Chapter 4..... 0Document6 pagesChapter 4..... 0Margarita Limon BalunesNo ratings yet
- Answer Key Nur 145 Cmca LecDocument11 pagesAnswer Key Nur 145 Cmca LecMargarita Limon Balunes0% (1)
- Articles Cons of AbortionDocument2 pagesArticles Cons of AbortionMargarita Limon BalunesNo ratings yet
- Nur 145 Lec SasDocument22 pagesNur 145 Lec SasMargarita Limon BalunesNo ratings yet
- Case Study FinalDocument8 pagesCase Study FinalMargarita Limon BalunesNo ratings yet
- Diet DiaryDocument5 pagesDiet DiaryMargarita Limon BalunesNo ratings yet