Professional Documents
Culture Documents
Practical Food Tests
Uploaded by
paulamjinga3Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Practical Food Tests
Uploaded by
paulamjinga3Copyright:
Available Formats
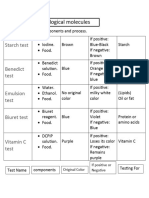
FOOD TEST
PART1: TESTING FOR SIMPLE SUGARS
Tube Sample Initial Colour Colour after Conclusion
heat
1 Water Blue Blue The sample does not
contain simple sugars since
colour remained blue.
2 Glucose Blue Brick red Colour changed from blue
to brick red meaning
simple sugars are present.
3 Starch Blue Blue Sample remained blue
meaning it does not contain
simple sugars.
4 Orange juice Blue Orange Colour for orange juice
sample changed from blue
to orange hence sample is
positive for simple sugars.
5 Regular soda Blue Orange Regular soda contains
simple sugars since colour
changed from blue to
orange.
6 Diet soda Blue Blue Diet soda does not contain
simple sugars since colour
remained blue.
7 Unknown Blue Blue This sample does not
contain simple sugars since
colour remained blue.
PART 2: TESTING FOR STARCH
(Lubol’s Reagent: IKI Solution turns dark blue/ black in the presence of starch)
RESULTS
Tube Sample Initial colour Colour after Conclusion
IKI
1 Water Dark brown Dark brown The sample does not
contain starch
2 Glucose Dark brown Dark brown There is no starch in this
sample
3 Starch Dark brown Blue-black Starch is present since the
colour changed from dark
brown to blue-black.
4 Orange juice Dark brown Dark brown Starch is absent
5 Regular soda Dark brown Dark brown The sample does not
contain starch
6 Diet soda Dark brown Dark brown There is no starch present
7 Unknown Dark brown Dark brown Starch test tested negative
meaning it’s not present in
this sample.
PART 3: TESTING FOR LIPIDS
A: PAPER TEST
RESULTS
Tub Sample Appearance after drying Conclusion
e
1 Water No translucent mark Lipids are not present
2 Oil The paper had translucent mark Lipids are present
3 Cream The paper had translucent mark Lipids are present
4 Unknown The paper had translucent mark Lipids are present
B: SUDAN IV TEST
RESULTS
Tube Sample Appearance after Sudan іv test Conclusion
1 Water The water remains clear Lipids are not present
2 Oil The oil turns red. Lipids are present
3 Cream The cream turns red. Stains lipid red Lipids are present
4 Unknown Red colour observed Lipids are present
PART 4: TESTING FOR PROTEINS
BIURET’S REAGENT TURNS DARK BLUE-PURPLE IN PRESENCE OF
PEPTIDE BONDS.
RESULTS
Tube Sample Initial colour Colour after Conclusion
biuret
1 Water Blue Blue Protein is absent
2 Starch Blue Blue Protein is absent
3 Egg albumen Blue Purple This sample contain protein
4 Glucose Blue Blue Protein is absent
5 Regular soda Blue Blue Protein is not present
6 Unknown Blue Purple This sample contain protein
UNKNOWN
By looking at the collected data, the union or fusion of major molecules present in the
mixture which is unknown are lipids and proteins since the unknown mixture tested
positive for lipids and proteins.
QUESTIONS
1. The monomers that make up carbohydrates
-Monosaccharides are the monomers that make up carbohydrates which are
known as simple sugars which cannot be further broken down into smaller units
or smaller sugars. These Monosaccharides are the ones that combine to form
complex carbohydrates such as oligosaccharides, polysaccharides and
disaccharides.
-Monosaccharides are of three types, these are;
(a) Trioses- Trioses contain three atoms of carbon. They include
dihydroxyacetone and glyceraldehyde.
(b) Hexoses- Hexoses contain six atoms of carbon. Examples of hexoses are
Fructose, galactose and glucose.
(c) Pentoses- They contains five atoms of carbon. They include deoxyribose
and ribose.
-If Monosaccharides are joined together, they form much complex
carbohydrates by a process which is known as Dehydration synthase. In this
process, a water molecule is removed from two Monosaccharides, and the
Monosaccharides that remain are fused or bonded by a covalent bond together.
-Below is a list of carbohydrates and their monosaccharides components:
(і) Fructose- Insect monosaccharide which is found in honey and fruits
(іі) Glucose- This is a common monosaccharide which is found in different
kinds in foods such as vegetables and fruits.
(ііі) Starch- This is a polysaccharide which is made of many molecules of
glucose. Plants store carbohydrates in this form.
(іv) Maltose- Maltose is a disaccharide which is produced by the breaking
down of starch by the process of digestion. It is made up of two glucose
molecules. It is found in plant grains.
(v) Sucrose- This is a disaccharide which is made up of fructose and glucose
bonded together.
(vі) Galactose- It is found in milk and some daily products. It is a
monosaccharide.
(vіі) Glycogen- It is made of many glucose molecules hence it is a
polysaccharide. Animals store carbohydrates in form of this polysaccharide. It is
mainly stored in animal muscles and the liver.
(ііx) Lactose- it is made up of two forms of carbohydrates which are galactose
and glucose hence it is a disaccharide. It is mainly found in milk and other dairy
products.
2. The monomers that form up proteins
-Amino acids are the monomers that form up proteins. Amino acids are of 20
types that are mostly found in proteins. Each amino acid have a structure which
is different from the other and this gives each protein it’s unique properties.
-Amino acids are bonded together by peptide bonds to form proteins and these
peptide bonds are produced by a reaction which is called dehydration synthesis.
This reaction removes a water molecule from two main amino acids. This
reaction can be a reversible one through a reaction of hydrolysis which adds a
molecule of water to a peptide bond and break it.
-Below is a list of amino acids and the protein that they are found in:
(і) Arginine- It is found in elastin, collagen and immunoglobulin.
(іі) Alamine- It is found in in many proteins like myosin, keratin and
hemoglobin.
(ііі) Valine- It is found in myosin, Hemoglobin and other proteins found in the
muscles.
(іv) Tryosin- This amino acid is found in epinephrine, thyroxin and other
proteins.
(v) Tryptophan- It is found in melatonin, serotonin and other proteins.
(vі) Tubeonine- It is found in collagen, elastin and other proteins.
(vіі) Serine- It is found in immune globulins, enzymes and other proteins.
(ііx) Proline- It is found in elastin, keratin and collagen.
(іx) Phenylalanine- It is found in phenylalanine hydroxylase which is an
enzyme that convert phenylalanine to tyrosine.
(x) Lysine- It is found in elastin, enzymes and collagen.
(xі) Leucine- It is found in myosin and hemoglobin.
(xіі) Isoleucine- It is found in myosin, hemoglobin and other proteins.
(xііі) Histioine- It is found in hemoglobin, Cytochrome C and histamine.
(іvx) Glycine- It is found in elastin, hemoglobin and collagen.
(vx) Cysteine- It is found in insulin, antibodies and keratin.
(xvі) Aspartic acid- It is found in hormones, enzymes and neurotransmitters.
(xvіі) Arginine- It is found in elastin, collagen and immunoglobulin.
3. Which test could you use to distinguish diet and regular soda? What would
the test detect?
-To distinguish regular and diet soda, iodine test for starch is used. This test will
detect the presence of sugar in soda. Diet soda does not contain sugars whereas
regular soda contains sugars. During the test, if the soda remains orange-brown,
then it is diet soda and if the soda turns a deep blue-black colour, it means its
regular soda.
-One can use refractometer which is a device that measures refractive index of a
liquid. This refractive index of a liquid is affected by its concentration of
dissolved solids. Diet soda will have a lower refractive index than regular soda
since sugar is a dissolved solid. If the refractive index on the scale is way above
1.35 then the soda is regular whilst if the refractive index is below 1.35, then its
diet soda.
4. What is the Biuret test actually detecting? Be as specific as possible.
-Biuret test detects the presence of two or more peptide bonds specifically. The
peptide bonds are those that join amino acids together forming proteins. This
biuret test works by producing a complex with the copper ions in the biuret
reagent.
-This complex turns the solution to a purple colour. The intensity of the purple
colour is proportional to the number or quantity of peptide bonds in the
solution.
5. Each test included a sample that was just water. Why it is important to
include a water only sample for each test?
-For these two reasons that’s why water is included:
(і) To control for reagent test’s purity- Test reagents are those chemicals that
are used to detect the presence of a particular substance or the absence of a
particular substance in a food sample. When the reagents are not pure, false
results that are either positive or negative can be given. Through the
inclusion of a water-only sample, this will make sure that the test reagents are
not giving results that are not correct.
(іі) To regulate the effects of other substances in a food sample- Food is a
complex mixture of many different kinds of substances. A lot of these
substances may interfere or disrupt the reagents of the test and thus false
results may be given. Through the inclusion of a water-only sample, some
substances present in the food sample that will be interfering with the test can
easily be identified.
References
Ashurst PR. Food Additions. 4th Ed Blackwell Publishings, 2013
Bruce, A., Alexander, J., Julien, L. and Keith, R. Biochemistry: Concepts and Compositions.
7th Edition, 2017.
Compendium of Methods for the Microbiological Examination of Foods (Microbiology
Compendium). 5th Ed. US. Food and Drug Administration, 2019.
David, L. N and Michael, M. Lehninger Principles of Biochemistry. 8th Edition, 2023.
Jeremy, M. B, John, L. T and Lubert, S. Biochemistry 9th Edition, 2022.
Mc Gee H. Food Chemistry. 5th Ed. W.W. Norton Company 2014.
Nielsen SS. Food Analysis. 5th Ed. Springer, 2017.
Official methods of Analysis of the Association of Official Analytical Chemists (AOAC).
22nd Ed. AOAC International, 2020.
You might also like
- LAB REPORT EXP 1 G3 Dalila - Faris - Imannina - Farhana Sabuddin - ElianaDocument9 pagesLAB REPORT EXP 1 G3 Dalila - Faris - Imannina - Farhana Sabuddin - ElianaKhairul Salam HasinorNo ratings yet
- Starch Digestion: Identifying Starch and Reducing Sugars in SeedsDocument5 pagesStarch Digestion: Identifying Starch and Reducing Sugars in SeedsFathiah NhNo ratings yet
- Qualitative Tests for ProteinDocument11 pagesQualitative Tests for ProteinElla Nicole TaljaNo ratings yet
- Lab 7 RadDocument4 pagesLab 7 RadHanrey AveryNo ratings yet
- Running Head: Food Test Lab Report 1Document6 pagesRunning Head: Food Test Lab Report 1Jun Hong Tee100% (2)
- Test For Carbohydrates PracticalDocument18 pagesTest For Carbohydrates PracticalESTHER WONG TZE YIING -No ratings yet
- Zoo 120.1 - Experiment 6Document3 pagesZoo 120.1 - Experiment 6Rey Malvin SG PallominaNo ratings yet
- Identify Biochemicals in Food and Pure SamplesDocument5 pagesIdentify Biochemicals in Food and Pure SamplesSteven KhanNo ratings yet
- Bio Lab Report (G2) PDFDocument10 pagesBio Lab Report (G2) PDFAina NabihaNo ratings yet
- Laboratory Report Lab 1 Starch Analysis: Sbk3023 Food ScienceDocument6 pagesLaboratory Report Lab 1 Starch Analysis: Sbk3023 Food ScienceNur Hana SyamsulNo ratings yet
- Experiment 1Document6 pagesExperiment 1Nur Hana SyamsulNo ratings yet
- Lab Report Group 1Document9 pagesLab Report Group 1Rachel ButilNo ratings yet
- Inquiry in Context (Test For Starch)Document3 pagesInquiry in Context (Test For Starch)BSNo ratings yet
- Exp. 8 WorksheetDocument11 pagesExp. 8 WorksheetChristina mikaela CabusaoNo ratings yet
- Qualitative food tests detect biomoleculesDocument5 pagesQualitative food tests detect biomoleculesZachary MedwinterNo ratings yet
- Benedict TestDocument3 pagesBenedict TestArwin GraaffNo ratings yet
- Tests for Organic MoleculesDocument7 pagesTests for Organic MoleculesICAMisterPNo ratings yet
- Food Test Detects CompoundsDocument4 pagesFood Test Detects CompoundsLim Chun Yue100% (1)
- Biology Food Tests:: Starch TestDocument1 pageBiology Food Tests:: Starch TestsalmasomaNo ratings yet
- Molecules of Life Lab ReportDocument37 pagesMolecules of Life Lab ReportAdelia HanaNo ratings yet
- Chem PorjectDocument3 pagesChem PorjectthedabberiscoolNo ratings yet
- Practical 5 PDFDocument17 pagesPractical 5 PDFdoblekerpiyushNo ratings yet
- Food Tests Reveal Drink's NutrientsDocument6 pagesFood Tests Reveal Drink's NutrientsSasha SweetNo ratings yet
- Identification of Biological Molecules in FoodDocument13 pagesIdentification of Biological Molecules in FoodNurul Ain AfiqahNo ratings yet
- Lab Report: Using The Food Lab: Testing For Vitamin CDocument12 pagesLab Report: Using The Food Lab: Testing For Vitamin Ckim gopaulNo ratings yet
- Postlab Discussion Expt 2 Detection of Carbohydrates, Proteins and LipidsDocument19 pagesPostlab Discussion Expt 2 Detection of Carbohydrates, Proteins and LipidsDale Miko SanchezNo ratings yet
- Food Tests Transcript: Cambridge IGCSE ™Document6 pagesFood Tests Transcript: Cambridge IGCSE ™Night suitNo ratings yet
- Chemistry ProjectDocument12 pagesChemistry ProjectPunit Mukherjee100% (1)
- Food Tests in Biology AISDocument1 pageFood Tests in Biology AISMohamed YanishNo ratings yet
- Biology Lab ReportDocument4 pagesBiology Lab Reporttinsae tewodrosNo ratings yet
- Test For Glucose and Albumin Laboratory in BiochemistryDocument10 pagesTest For Glucose and Albumin Laboratory in BiochemistryJe KirsteneNo ratings yet
- Procedure & Result Bio270Document5 pagesProcedure & Result Bio270Nurmalisa HerwanyNo ratings yet
- Grade 7 Science QuesDocument47 pagesGrade 7 Science QuesSai Sumedh ChellapillaNo ratings yet
- Nutrient Analysis - Lab Report - BiochemDocument3 pagesNutrient Analysis - Lab Report - BiochemdzdooNo ratings yet
- Biomolecules in My Food Laboratory Procedures Test For LipidDocument2 pagesBiomolecules in My Food Laboratory Procedures Test For LipidAriel DayritNo ratings yet
- Food TestsDocument4 pagesFood Testsrchataika863No ratings yet
- Samples Color of Indicator Nature of SampleDocument3 pagesSamples Color of Indicator Nature of SampleGloryvill FalsisNo ratings yet
- Abhi ChemistryDocument17 pagesAbhi ChemistryAbhishek MahapatroNo ratings yet
- Biomolecules Lab ReportDocument2 pagesBiomolecules Lab Reportapi-372144619No ratings yet
- J. Ramdath - Lab 1 BioDocument4 pagesJ. Ramdath - Lab 1 BioJosh RamdathNo ratings yet
- Identify Biomolecules in Food SamplesDocument4 pagesIdentify Biomolecules in Food SamplesAkeisha King0% (1)
- Biochemistry Assessment1Document2 pagesBiochemistry Assessment1Xandra ArticuloNo ratings yet
- Abnormal urine constituents detectionDocument2 pagesAbnormal urine constituents detectionAries DocNo ratings yet
- Food tests investigation resultsDocument3 pagesFood tests investigation resultsNeelam HanifNo ratings yet
- Experiment Reveals Lipid PropertiesDocument9 pagesExperiment Reveals Lipid PropertiesRameesh IshakNo ratings yet
- Chapter 4 Biological MoleculesDocument1 pageChapter 4 Biological MoleculesJumana ElkhateebNo ratings yet
- Shugarse and StarcheDocument3 pagesShugarse and Starchejohannaquino992No ratings yet
- Testing For Biological MoleculesDocument7 pagesTesting For Biological MoleculesAqeelah IsaacsNo ratings yet
- Chemistry Project Class 12Document12 pagesChemistry Project Class 12mihir khabiyaNo ratings yet
- YIH YEE CHONG - Summative Assessment - Unit 2 Saliva ExperimentDocument6 pagesYIH YEE CHONG - Summative Assessment - Unit 2 Saliva ExperimentYIH YEE CHONGNo ratings yet
- Biology Report (Experiment 1)Document2 pagesBiology Report (Experiment 1)djash9267% (3)
- Lab 11 BIOCHEM REPORTDocument8 pagesLab 11 BIOCHEM REPORTAna LuisaNo ratings yet
- Food Test LabDocument4 pagesFood Test LabTanieka PowellNo ratings yet
- Exercise 2: Biomolecules: Personal InformationDocument5 pagesExercise 2: Biomolecules: Personal InformationRichard Anthony CardenasNo ratings yet
- Biochemical food testsDocument8 pagesBiochemical food testsSyafiqah SabriNo ratings yet
- Worksheet Module 2Document4 pagesWorksheet Module 2YuraNo ratings yet
- Enzymes Lab ReportDocument8 pagesEnzymes Lab ReportMaizamNo ratings yet
- Lab ReportDocument4 pagesLab Reportapi-289126506No ratings yet
- State of Childhood Obesity 10 14 20 Final WEB PDFDocument48 pagesState of Childhood Obesity 10 14 20 Final WEB PDFWSETNo ratings yet
- Food and Nutrition Research Institute (FNRI) Food Guide PyramidDocument28 pagesFood and Nutrition Research Institute (FNRI) Food Guide PyramidWincy SalazarNo ratings yet
- Foods: Nanoemulsions As Edible Coatings: A Potential Strategy For Fresh Fruits and Vegetables PreservationDocument17 pagesFoods: Nanoemulsions As Edible Coatings: A Potential Strategy For Fresh Fruits and Vegetables PreservationAhmad Anas Nagoor GunnyNo ratings yet
- 0995 DUK - MealPlanner 1500 Veggie B 11 - 3 - 21Document2 pages0995 DUK - MealPlanner 1500 Veggie B 11 - 3 - 21PRAVASH KUMAR SINGHNo ratings yet
- Grade 9 Lo3Document14 pagesGrade 9 Lo3Joyzann Cellan100% (1)
- Outline Format Problem SolutionDocument2 pagesOutline Format Problem SolutionSofia Torres FlorezNo ratings yet
- Iron Deficiency Anemia Causes, Symptoms and PreventionDocument11 pagesIron Deficiency Anemia Causes, Symptoms and PreventionDwi AgustinNo ratings yet
- Junk food facts and effects on healthDocument2 pagesJunk food facts and effects on healthMagdi SisillaNo ratings yet
- (M.SC Thesis (Agronomy) Final - Dushyant)Document118 pages(M.SC Thesis (Agronomy) Final - Dushyant)somnath nayakNo ratings yet
- The Local Food Environment and Diet. A Systematic ReviewDocument16 pagesThe Local Food Environment and Diet. A Systematic ReviewJosue Montiel AvilezNo ratings yet
- Cutting Meal Plan 210 230 Pound ManDocument2 pagesCutting Meal Plan 210 230 Pound ManGeorge VNo ratings yet
- Grade 7 BiologyTextbookDocument153 pagesGrade 7 BiologyTextbook소창주No ratings yet
- Jacabanas Ba 107 Assignment#1Document4 pagesJacabanas Ba 107 Assignment#1Jessuel Larn-epsNo ratings yet
- 151 - Effects of Exercise On Immuine Function - Gleeson 2015Document6 pages151 - Effects of Exercise On Immuine Function - Gleeson 2015Rahil PatelNo ratings yet
- Diabetes Teaching PlanDocument5 pagesDiabetes Teaching PlanArsha SashaNo ratings yet
- Nursing Care Plan for Post-Surgical Bleeding RiskDocument10 pagesNursing Care Plan for Post-Surgical Bleeding RiskRyan MirandaNo ratings yet
- De Cuong On Tap HKI Lop 7Document30 pagesDe Cuong On Tap HKI Lop 7- Khoa NN- ĐHKTKTCN Đặng Thị Thanh HươngNo ratings yet
- Week 16 Global Food SecurityDocument5 pagesWeek 16 Global Food Securitydonnasison0929No ratings yet
- # of Carbons Aldose Ketose: Carbons (Triose) - D. Glyceraldehyde and L. Glyceraldehyde. Notice The Positions of Both OHDocument7 pages# of Carbons Aldose Ketose: Carbons (Triose) - D. Glyceraldehyde and L. Glyceraldehyde. Notice The Positions of Both OHAngel CalambaNo ratings yet
- 3.dairy FarmingDocument14 pages3.dairy FarmingRakesh guptaNo ratings yet
- IGCSE Coordinated Sciences Animal NutritionDocument10 pagesIGCSE Coordinated Sciences Animal NutritionSeonaid McDonaldNo ratings yet
- Chapters 19 - 20 - 21Document51 pagesChapters 19 - 20 - 21Ghada AhmedNo ratings yet
- IYCF Book Final 30-01-2018Document96 pagesIYCF Book Final 30-01-2018Hafeez SaleemiNo ratings yet
- Low FODMAP Diet FODMAP Foods Nowhealth 1Document2 pagesLow FODMAP Diet FODMAP Foods Nowhealth 1MiraNo ratings yet
- LQH Lnap 2014-2016Document5 pagesLQH Lnap 2014-2016api-24692675980% (5)
- Science 8 Summative Exam Q4Document4 pagesScience 8 Summative Exam Q4Kelvin Jason ArellanoNo ratings yet
- Anorexia Nervosa InfographicDocument1 pageAnorexia Nervosa Infographicapi-518183280No ratings yet
- Microbiology of Fermented Milk Product Ass Bscag4Document3 pagesMicrobiology of Fermented Milk Product Ass Bscag4Shailesh Singh PatelNo ratings yet
- Nutrition's Role in Celiac Disease DevelopmentDocument4 pagesNutrition's Role in Celiac Disease DevelopmentKajee GrantNo ratings yet
- Nasogastric Feeding GuidelineDocument25 pagesNasogastric Feeding GuidelineElse FashiosNo ratings yet