Professional Documents
Culture Documents
ENZYMES
Uploaded by
ariansofia10310 ratings0% found this document useful (0 votes)
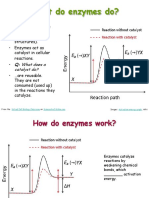
9 views5 pagesEnzymes are protein catalysts that speed up biochemical reactions without being consumed. They are responsible for processes in living organisms and each cell contains thousands of different enzymes. Enzymes work by lowering the activation energy of reactions, allowing reactions to proceed millions of times faster than uncatalyzed reactions. Enzymes can be made of just protein or can have additional non-protein parts called cofactors that help the enzyme function. Cofactors are often vitamins, metal ions, or small organic molecules that bind to the protein part of enzymes called the apoenzyme to form the active enzyme called the holoenzyme. Enzymes are classified based on the type of reaction they catalyze.
Original Description:
biochem review enzyme #1
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentEnzymes are protein catalysts that speed up biochemical reactions without being consumed. They are responsible for processes in living organisms and each cell contains thousands of different enzymes. Enzymes work by lowering the activation energy of reactions, allowing reactions to proceed millions of times faster than uncatalyzed reactions. Enzymes can be made of just protein or can have additional non-protein parts called cofactors that help the enzyme function. Cofactors are often vitamins, metal ions, or small organic molecules that bind to the protein part of enzymes called the apoenzyme to form the active enzyme called the holoenzyme. Enzymes are classified based on the type of reaction they catalyze.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
9 views5 pagesENZYMES
Uploaded by
ariansofia1031Enzymes are protein catalysts that speed up biochemical reactions without being consumed. They are responsible for processes in living organisms and each cell contains thousands of different enzymes. Enzymes work by lowering the activation energy of reactions, allowing reactions to proceed millions of times faster than uncatalyzed reactions. Enzymes can be made of just protein or can have additional non-protein parts called cofactors that help the enzyme function. Cofactors are often vitamins, metal ions, or small organic molecules that bind to the protein part of enzymes called the apoenzyme to form the active enzyme called the holoenzyme. Enzymes are classified based on the type of reaction they catalyze.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 5
GENERAL CHARACTERISTICS OF - Catalysts or enzymes are responsible for
ENZYMES different biological changes.
EARLY ENZYMES DISCOVERIES
- They make up the largest and most
ENZYMES
highly specialized class of proteins. JON JAKOB BERZELIUS – 1835 Swedish
- Is a compound, usually a protein, that acts - The catalytic efficiency of enzyme is so Chemist, termed their chemical action – catalytic.
as a catalyst for biochemical reaction. high that per mole is around 10,000 to
- Each cell in the human body contains 10,000,000 moles of substance per JAMES B. SUMMER of Cornell +University –
thousands of different enzymes because minute. Isolate and crystallize the enzymes urease from
almost every reaction in the cell requires - The catalytic action of enzymes is the key the jack bean.
its own specific enzyme. to their importance, for by facilitating JOHN H. NORTHROP and WENDELL M.
- Enzymes cause cellular reactions to occur chemical changes, enzymes make STANLEY – They discovered a complex
millions of times faster than corresponding possible the continuous replacement procedure for isolating and purifying pepsin.
uncatalyzed reactions. and renewal processes of all living
- As catalyst, enzymes are not consumed organisms. ENZYME STRUCTURE
during the reaction but merely help in the - The word ENZYME comes from a Greek
A. Simple Enzymes
reaction occur more rapidly. word EN, which means “IN” and ZYME,
which means “YEAST”. Is an enzyme composed only of
- Are chemical substance which hastens
protein (amino acid chains)
chemical reaction without being affected in
YEAST B. Conjugated Enzymes
the process.
- Are secreted by living cells and are Is an enzyme that has a nonprotein
Long before, yeast is used in the production of
complex organic chemical compounds with part in addition to protein part.
bread and alcoholic beverages.
definite structure. APOENZYME is the protein part of
- Like the inorganic catalysts, enzymes have - Yeast reaction to sugar produces the a conjugated enzymes.
the remarkable property of speeding up carbon dioxide gas that causes the bread COFACTOR Is the nonprotein part
chemical reactions without being to rise. of the conjugated enzymes.
themselves affected in the process. - Fermentation of sugars in fruit juice using HOLOENZYME Is the
the yeast enzymes produces alcoholic biochemically active conjugated
As a result, they can be use over and over beverages. enzymes produced from an
again.
apoenzyme and a cofactor.
Apoenzyme + Cofactor = Holoenzymes
Why do apoenzyme need cofactor? - CONENZYME is a small organic molecule PROSTHETIC GROUP – The nonprotein
that serves as a cofactor in conjugated component, tightly bound to the apoenzyme by
- Cofactors provide additional chemically
enzyme. covalent bonds is called a prosthetic group.
reactive functional group besides those
- COENZYME is synthesized within the
present in the amino acid side chains of NOMENCLATURE AND CLASSIFICATION
human body using building blocks from the
apoenzymes. OF ENZYMES
nutrients.
APOENZYME and HOLOENZYME o Most often, one of these building
blocks is a B vitamin or B
- The enzyme without its non-protein moiety vitamin derivatives.
is termed as apoenzyme and it is inactive. o Vitamins must be obtained
- Holoenzyme is an active enzyme with its through dietary intake.
nonprotein component. - Many cofactors are permanently bonded,
TWO BROAD CATEGORIES OF COFACTOR: via covalent bond, to the amino acids
portion of enzymes.
A. Simple Metal Ions
- Breaking the covalent bond deactivates Enzymes – are most commonly named by using
B. Small Organic Molecules
the enzymes. a system that attempts to provide information
METAL IONS COFACTOR o Ex. Coenzymes FAD which has about the function (rather than the structure) of
riboflavin (a B vitamin) the enzymes.
- Include Zn, Mg, Fe, and Cu. o Coenzymes NAD which has niacin
- All metal ions must be supplied to human (a B vitamin) - The type of reaction catalyzed, and the
body through dietary mineral intake. substrate identify are focal points in
- Almost any type of diet will provide TYPES OF COFACTORS nomenclature.
adequate amounts of needed metallic - A substrate is the reactant in an enzyme-
COENZYMES – The nonprotein component, catalyzed reaction. The substrate is the
cofactors because they are needed in very
loosely bound to apoenzyme by non-covalent substances upon which the enzymes
small (trace) amounts.
bond. “acts”.
SMALL ORGANIC MOLECULES
Examples: vitamins or compound derived Three important aspects of the enzymes-naming
from vitamins. process are the following:
- The second category of cofactors, as a
group, are called COENZYMES.
1. The suffix ASE identifies the substance Enzymes – are grouped into six major classes CLASSIFICATION OF ENZYMES
as an enzyme. (EC Number Classification) by the International
a. Thus urease, surcease and lipase Union of Biochemists (I.U.R). On the basis of
are all enzymes designation. types of reactions, they catalyze.
b. The suffix IN is still found in the
PRINCIPLE OF THE INTERNATIONAL
names of some of the first enzymes
CLASSIFICATION
studied, many of which are
digestive enzymes. Each enzyme has classification number
i. Ex. Trypsin, chymotrypsin, consisting of four digits:
and pepsin.
Example EC: (2.7.1.1) HEXOKINASE
2. The type of reaction catalyzed by an
enzyme is often noted with a prefix. EC: (2.7.1.1) these components indicate the
a. An oxidase enzyme catalyzes an following groups of enzymes:
oxidation.
2 is class (TRANSFERASE) 1. OXIDOREDUCTASE – Is an enzyme that
b. A hydrolase enzyme catalyzes a
catalyzes an oxidation-reduction
hydrolysis reaction. 7 is subclass (TRASFER OF PHOSPHATE)
reaction.
3. The identity of the substrate is often
1 is sub subclass (ALCOHOL IS PHOSPHATE
noted in addition to the type of
ACCEPTOR)
reaction.
a. Enzymes names of this type include 1 specific name: ATP, D-HEXOSE-6-
glucose oxidase, pyruvate PHOSPHOTRANSFERASE (HEXOKINASE)
carboxylase, and succinate
dehydrogenase.
An organic oxidation reaction is an oxidation
b. In frequently, the substrate but not
that increases the number of C-O bonds and/or
the reaction types are given, as in
decreases the of C-H bonds.
the name’s urease and lactase.
c. In these names, the reaction An organic reduction reaction is a reduction that
involved is hydrolysis; urease decreases the number of C-O bonds and/or
catalyzes the hydrolysis of urea, increases the number of C-H bonds.
lactase the hydrolysis of lactose.
The oxidation-reduction are not independent Kinases which play a major role in metabolic Catalyze the hydrolysis of various bonds
processes but linked processes that must occur energy-production reactions, catalyzes the add water across a bond.
together. transfer of a phosphate group from adenosine
EXAMPLES:
triphosphate (ATP) give adenosine diphosphate
An oxidoreductase requires a coenzyme that is
(ADP) and phosphorylated products (a Protein Hydrolyzing Enzymes
oxidized or reduced as the substrate is reduced
phosphate containing an additional phosphate). (Peptidases)
or oxidized.
Carbohydrases (Amylase, Maltase,
EX. Lactase Dehydrogenase is an Lactase)
oxidoreductase that removes hydrogen atom Lipid Hydrolyzing Enzymes (Lipase)
from a molecule. Deaminases
2. TRANSFERASE – Is an enzyme that Phosphatases
catalyzes the transfer of a functional group
from one molecule to another.
BIOCHEMICAL ACTIVITY: 3. HYDROLASE – Is an enzyme that
catalyzes a hydrolysis reaction in which
Transfer a functional group (e.g., methyl or
the addition of a water molecule to a
phosphate) between donor and acceptor
molecule to a bond causes the bond to
molecules.
break.
EXAMPLES: 4. LYASES – Is an enzyme that catalyzes the
Hydrolysis Reactions – are central to the addition of a group to a double bond or the
Transaminases (ALT & AST) process of digestion. removal of a group to form a double bond
Phosphotransferases (Kinases) in a monomer that does not involve
Carbohydrates effect the breaking of glycosidic
Transmethylases bonds in oligo and polysaccharides. hydrolysis or oxidation.
Transpeptidases
Proteases effect the breaking of peptide linkages A dehydratase effects the removal of the
Transacylases
in protein. components of water from a double bond.
Two major subtypes of transferase are
Lipases effect the breaking of ester linkages in Hydratase effects the addition of the components
transaminases and kinases.
triacylglycerol’s. of water to a double bond.
Transaminase catalyzes the transfer of amino
BIOCHEMICAL ACTIVITY: BIOCHEMICAL ACTIVITY:
group from one molecule to another.
Cleave various bonds by means other BIOCHEMICAL ACTIVITY: BIOCHEMICAL ACTIVITY:
than hydrolysis and oxidation.
Catalyze isomerization changes within a Join two molecules with covalent bonds
Add water, ammonia or carbon dioxide
single molecule. catalyze reactions in which two chemical
across double bonds, or remove these
Carry out many kinds of isomerization: groups are joined (or ligated) with the use
elements to produce double bonds.
o L to D isomerization of energy from ATP.
EXAMPLES: Many kinds of isomerization EXAMPLES:
o L to D isomerization
Fumarase
o Mutase reactions (shifts of chemical Acetyl-CoA Carboxylase
Carbonic Anhydrase
groups) Glutamine Synthetase
Ligases (Synthetases)
EXAMPLES:
Catalyze ligation or joining of two
Isomerase substrates.
Mutase Require chemical energy (e.g., ATP)
5. ISOMERASE – Is an enzyme that
catalyzes the isomerization
(rearrangement of atoms) of a substrate 6. LIGASES – Is an enzyme that catalyzes
7. TRANSLOCASES – catalyzing the
in a reaction, converting it into a molecule the bonding together of two molecules into
translocation of hydrogen ions, inorganic
isomeric with itself. one with the participation of ATP.
cations and anions, amino acids,
There is only one reactant and one product in ATP involvement is required because such carbohydrates or other compounds.
reactions where isomerases are operative. reaction is generally energetically unfavorable,
Isomerases – are a general class of enzymes and they required the simultaneous input of
which convert a molecule from one isomer to energy obtained by a hydrolysis reaction in which
another. The general form of such a reaction is as ATP is converted to ADP.
follows: A-B – B-A.
You might also like
- Medicinal Plants - Properties, Uses and Production - Deepak Kumar Semwal PHDDocument338 pagesMedicinal Plants - Properties, Uses and Production - Deepak Kumar Semwal PHDpedropereza88No ratings yet
- Clinical Chemistry 2Document22 pagesClinical Chemistry 2Rubenne Miles ElagasNo ratings yet
- Enzyme and Enzyme KineticsDocument7 pagesEnzyme and Enzyme KineticsSam Jeffrey100% (2)
- Fermentation & Distillation: Er. Raghvendra SachanDocument22 pagesFermentation & Distillation: Er. Raghvendra SachanSantiagoNo ratings yet
- Enzymology Part 1-2Document12 pagesEnzymology Part 1-2Anya IgnacioNo ratings yet
- Decomposition of Organic Matter in WaterDocument6 pagesDecomposition of Organic Matter in WaterDivya Reddy100% (1)
- Principles of Animal Nutrition (PDFDrive)Document801 pagesPrinciples of Animal Nutrition (PDFDrive)mehtaorgautam100% (1)
- ENZYMESDocument9 pagesENZYMESariansofia1031No ratings yet
- Enzymes ReviewerDocument15 pagesEnzymes ReviewerAbby Dimalaluan OquendoNo ratings yet
- Biology FinalsDocument2 pagesBiology FinalsbhongskirnNo ratings yet
- Biochem Enzyme NotesDocument8 pagesBiochem Enzyme NotesBeatriz NideaNo ratings yet
- Chapter Iv EnzymesDocument6 pagesChapter Iv EnzymesJohn TacordaNo ratings yet
- Biochem Assignment 2Document5 pagesBiochem Assignment 2Hadia SajidNo ratings yet
- Enzymes and VitaminsDocument2 pagesEnzymes and VitaminsdarleneNo ratings yet
- Enzymes PreMed-1Document16 pagesEnzymes PreMed-1Muhammad AtirNo ratings yet
- C5 - Metabolism and Enzyme Part 1Document20 pagesC5 - Metabolism and Enzyme Part 1Daniel LohNo ratings yet
- Aileen JoyDocument11 pagesAileen JoyHardy ValentinNo ratings yet
- Immunoglobulins: Immunoglobulins or Antibodies Are Defence Proteins Present in The Blood ToDocument10 pagesImmunoglobulins: Immunoglobulins or Antibodies Are Defence Proteins Present in The Blood ToAbhishek ThakralNo ratings yet
- ZOO 103 Lecture 09 19 ProteinsDocument12 pagesZOO 103 Lecture 09 19 ProteinsKaelyn MontefalconNo ratings yet
- Q2 Genbio 1 1Document9 pagesQ2 Genbio 1 1GABRIEL LOUIS GUANONo ratings yet
- Enzyme: Holoenzyme-BiochemicallyDocument8 pagesEnzyme: Holoenzyme-BiochemicallyStefanie LucresiaNo ratings yet
- Metabolisme - Kuliah 3 (Compatibility Mode)Document9 pagesMetabolisme - Kuliah 3 (Compatibility Mode)uniNo ratings yet
- Enzyme Brochure Gen Chem PTDocument2 pagesEnzyme Brochure Gen Chem PTAdrielNo ratings yet
- Biochem EnzymesDocument6 pagesBiochem EnzymesvalenciajohannahNo ratings yet
- Enzymes NotesDocument117 pagesEnzymes NotesRichel Ameryl DasocNo ratings yet
- Enzymes: Natures Chemical WorkforceDocument17 pagesEnzymes: Natures Chemical WorkforceMatt BarnardNo ratings yet
- 3.07: Proteins - Types and Functions of ProteinsDocument2 pages3.07: Proteins - Types and Functions of Proteinsdeepanshujadon28No ratings yet
- Gen Chem Peta 2nd QuarterDocument5 pagesGen Chem Peta 2nd QuarterJhames HarveyNo ratings yet
- Enzymes Course BioprocessDocument76 pagesEnzymes Course BioprocessiantitosimanjuntakNo ratings yet
- Proteins and EnzymesDocument2 pagesProteins and EnzymesAww AddNo ratings yet
- Enzyme: Specific Proteins That Catalyze Biochemical: Constituents of Enzyme MoleculeDocument6 pagesEnzyme: Specific Proteins That Catalyze Biochemical: Constituents of Enzyme MoleculeAnya IgnacioNo ratings yet
- Lesson 4 - ENZYMESDocument10 pagesLesson 4 - ENZYMESJhana SamsonNo ratings yet
- Cchem Lec Trans 1 1Document6 pagesCchem Lec Trans 1 1MJ ArboledaNo ratings yet
- A. What Are Enzymes?: Enzymes Prepared by La Vera U. Sombito Natural Sciences Department, CAS - USLSDocument4 pagesA. What Are Enzymes?: Enzymes Prepared by La Vera U. Sombito Natural Sciences Department, CAS - USLSJacqueline Rose Alipo-onNo ratings yet
- Enzymes: Learning ObjectivesDocument19 pagesEnzymes: Learning ObjectivesIyappan SubramaniNo ratings yet
- Bio Metabolisme EnzimDocument3 pagesBio Metabolisme EnzimGiska AliyaNo ratings yet
- Enzymes and VitaminsDocument19 pagesEnzymes and VitaminsJoanna Marie TulioNo ratings yet
- c1.1 EnzymesDocument36 pagesc1.1 Enzymeshwqhd asjdhuaNo ratings yet
- 231 Lecture 3Document12 pages231 Lecture 3Rana AbdullahNo ratings yet
- Chem 113 - Week 2 - EnzymesDocument5 pagesChem 113 - Week 2 - EnzymesFormosa G.No ratings yet
- PhmarmacogDocument8 pagesPhmarmacogMichelle CasilangNo ratings yet
- Week 9 - EnzymesDocument5 pagesWeek 9 - Enzymesjvlegaspi7463valNo ratings yet
- EnzymesDocument14 pagesEnzymesAlakesh Coldplay KalitaNo ratings yet
- Enzymes Are Proteins A Definitive Guide of 4000+ Words (Updated)Document15 pagesEnzymes Are Proteins A Definitive Guide of 4000+ Words (Updated)mammmmNo ratings yet
- Chapter 5 - Metabolism and EnzymesDocument24 pagesChapter 5 - Metabolism and EnzymesbubabozN aOIDHao8nxNo ratings yet
- CEnzymesDocument8 pagesCEnzymesCatherine RajanNo ratings yet
- Chapter 6Document48 pagesChapter 6Anupa GhoseNo ratings yet
- Material Complementario para Conferencia 1 Pags Capitulo 6 Lehninger 2013Document9 pagesMaterial Complementario para Conferencia 1 Pags Capitulo 6 Lehninger 2013Gabriela GarciaNo ratings yet
- Enzymes: 6.1 An Introduction To EnzymesDocument54 pagesEnzymes: 6.1 An Introduction To EnzymesBryan R. MenaNo ratings yet
- MCQs Exam MedDocument187 pagesMCQs Exam Medgeddy D.No ratings yet
- Chapter 5Document7 pagesChapter 5missmirachannel1No ratings yet
- Enzymes Part 1Document5 pagesEnzymes Part 1Mar LagmayNo ratings yet
- Clinical Chemistry IntroductionDocument4 pagesClinical Chemistry IntroductionPrecious PerniaNo ratings yet
- Unit 3 A EnzymesDocument3 pagesUnit 3 A EnzymesseecatpNo ratings yet
- Bio 2Document6 pagesBio 2Jaylene Kaye CabarrubiasNo ratings yet
- MetabolismDocument3 pagesMetabolismFans TimeNo ratings yet
- Module 8: Enzymes & Metabolic Pathways MetabolismDocument4 pagesModule 8: Enzymes & Metabolic Pathways MetabolismThiody Hope Mongas100% (2)
- Enzymes: Overview Enzyme: Structure: Small Organic Molecules Inorganic IonDocument19 pagesEnzymes: Overview Enzyme: Structure: Small Organic Molecules Inorganic IonUltima PhaseNo ratings yet
- EnzymesDocument25 pagesEnzymesmainakb2003No ratings yet
- Enzymes Part 2Document3 pagesEnzymes Part 2Mar LagmayNo ratings yet
- Lec1 - CLINICAL ENZYMOLOGYDocument5 pagesLec1 - CLINICAL ENZYMOLOGYClaire GonoNo ratings yet
- Energy and Enzymes IIDocument22 pagesEnergy and Enzymes IIapi-418176886No ratings yet
- Enzymes 1Document20 pagesEnzymes 1Saad ArsalanNo ratings yet
- Nature by NumbersDocument2 pagesNature by Numbersariansofia1031No ratings yet
- BLOODDocument4 pagesBLOODariansofia1031No ratings yet
- QUIZ No.2Document4 pagesQUIZ No.2ariansofia1031No ratings yet
- CPHM LAB Vital SignsDocument1 pageCPHM LAB Vital Signsariansofia1031No ratings yet
- CPAR Lesson 3Document3 pagesCPAR Lesson 3ariansofia1031No ratings yet
- ACTIVITY 5 - EntrepDocument1 pageACTIVITY 5 - Entrepariansofia1031No ratings yet
- Mole RatioDocument2 pagesMole Ratioariansofia1031No ratings yet
- Act 5Document1 pageAct 5ariansofia1031No ratings yet
- CPH Survey Tool-1Document2 pagesCPH Survey Tool-1ariansofia1031No ratings yet
- Program Book 希腊 打印版10Document88 pagesProgram Book 希腊 打印版101150810876No ratings yet
- ButanolDocument2 pagesButanolVanNo ratings yet
- ( ) Chemistry (Theory) : (Zym© (Av G Kêq O A (Yh$V A H$Document16 pages( ) Chemistry (Theory) : (Zym© (Av G Kêq O A (Yh$V A H$Ravneet KaurNo ratings yet
- Biology Chapter 3 EnzymesDocument4 pagesBiology Chapter 3 EnzymesAhmedNo ratings yet
- Chemical Engineering Department: College of Technology University of San Agustin Iloilo CityDocument13 pagesChemical Engineering Department: College of Technology University of San Agustin Iloilo CityReynee Shaira Lamprea MatulacNo ratings yet
- SeliwanoffDocument4 pagesSeliwanoffmarianjeanine100% (1)
- H C O Naoh: Acids and BasesDocument48 pagesH C O Naoh: Acids and BasesddddddNo ratings yet
- In Electric and Electronic Applications: Flame RetardantsDocument40 pagesIn Electric and Electronic Applications: Flame RetardantsHemanth KumarNo ratings yet
- JECFA - Mineral Oil - Monograph 2013Document2 pagesJECFA - Mineral Oil - Monograph 2013elenitabastosNo ratings yet
- Standardization of Asanabilvadi Taila and BilvadilehaDocument78 pagesStandardization of Asanabilvadi Taila and BilvadilehaOctavian PlayzNo ratings yet
- Wade 16Document41 pagesWade 16Carlos Javier Rodríguez ArroyoNo ratings yet
- GenchemDocument5 pagesGenchemYadnis Waters NaejNo ratings yet
- AP Bio PLUS - Ch. 1-7 Exam ReviewDocument5 pagesAP Bio PLUS - Ch. 1-7 Exam ReviewEmma HeathNo ratings yet
- A Study of The Deactivation of Low Loading Ni:Al2O3 Steam Reforming Catalyst by TetrahydrothiopheneDocument7 pagesA Study of The Deactivation of Low Loading Ni:Al2O3 Steam Reforming Catalyst by TetrahydrothiopheneVương Duy NghiêmNo ratings yet
- Stok TGL 30Document46 pagesStok TGL 30Riza FirdausNo ratings yet
- IV Anaesthetics SpreadsheetDocument2 pagesIV Anaesthetics SpreadsheetDonkeyManNo ratings yet
- DD Cen TS 13130-28-2005Document18 pagesDD Cen TS 13130-28-2005MladenMarkovicNo ratings yet
- Chemical ListDocument8 pagesChemical ListKodok TheexplorerNo ratings yet
- 2014-Article-FairyTALE - A High-Throughput TAL Effector Synthesis PlatformDocument7 pages2014-Article-FairyTALE - A High-Throughput TAL Effector Synthesis PlatformNghuijoNo ratings yet
- ASSESSMENT OF P-WPS OfficeDocument9 pagesASSESSMENT OF P-WPS OfficeDiether DavidNo ratings yet
- Laundry Cycle, and Stain RemovalDocument11 pagesLaundry Cycle, and Stain Removalsarthak rathiNo ratings yet
- Mallappa Kumara Swamy - Plant-Derived Bioactives - Chemistry and Mode of Action-Springer Singapore - Springer (2020)Document592 pagesMallappa Kumara Swamy - Plant-Derived Bioactives - Chemistry and Mode of Action-Springer Singapore - Springer (2020)Héctor Gómez YáñezNo ratings yet
- Chemistry SyllabusDocument7 pagesChemistry SyllabusSRISTI GUPTANo ratings yet
- Banana Peel Waste 1Document17 pagesBanana Peel Waste 1Gerald Mark CamayNo ratings yet
- Allen: Practical Organic Chemistry (Poc)Document4 pagesAllen: Practical Organic Chemistry (Poc)Uroosa AnsariNo ratings yet
- Ingrediente Prod EssenceDocument75 pagesIngrediente Prod EssenceMihaela MinaNo ratings yet