Professional Documents
Culture Documents
Distinction Tests 12th - Shobhit Nirwan
Uploaded by
modismit20060 ratings0% found this document useful (0 votes)
11 views1 pageOriginal Title
Distinction Tests 12th_Shobhit Nirwan
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
11 views1 pageDistinction Tests 12th - Shobhit Nirwan
Uploaded by
modismit2006Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
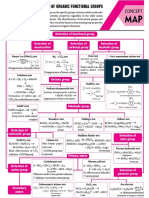
DISTINCTION TEST
Name Distinction Between Reagent Detection Remarks
3ᴼ- immidiate turbidity
Conc. HCL
Lucas Test 1ᴼ, 2ᴼ, 3ᴼ Alcohols 2ᴼ- turbidity after sometime OH Hatao Cl lagao + pani
Anhyd. AlCl3
1ᴼ- no turbidity
AgNO2 Test Haloalkane & Haloarene AgNO2 AgCl (white ppt) Cl hatao, NO 2 lagao
[Ag(NH3 ) 2 ]⁺ Aldehyde + [Ag(NH3)2]⁺ → RCOO⁻ + Ag↓
Tollen's Reagent Aldehyde & Ketone Alakaline Silver Mirror (Ag) But
medium Ketone + [Ag(NH3)2]⁺ → X
Aldehyde & Ketone
Fehling's Test 2Cu2+ + 4OH⁻ Red-Brown ppt (Cu2O) -
(Aromatic Aldehyde also)
Aldehyde & Ketone
CH3CO- + NaOH + I2 → CHI3 (yellow ppt)
Iodoform Test having minimum one CH3 NaOH + I2 Yellow ppt (CHI3)
CH3CHOH + NaOH + I2 → CHI3 (yellow ppt)
group
RCOOH & Phenol
NaHCO3 Test NaHCO3 CO2 RCOOH + NaHCO3 → RCOONa + CO2↑
RCOOH & Ester
Either RCHO
2,4 DNP Test Orange red
or Ketone
Double or triple bond
Bayer's Test Br2 / H2 O Decolorise C- + Br2 (Red) → -C(Br)-C(Br) (Colorless)
present in straight chain
Aliphatic and Aromatic β-napthol
Azo-Dye Test HNO2
1ᴼ Amines Orange dye (insoluble in water)
You might also like
- Organic Chemistry Fiitjee Flowcharts PDFDocument12 pagesOrganic Chemistry Fiitjee Flowcharts PDFTanishq VermaNo ratings yet
- AP Standard Data All PDFDocument963 pagesAP Standard Data All PDFSiva Kumar100% (1)
- What Is Your Road, Man?Document232 pagesWhat Is Your Road, Man?Oana AndreeaNo ratings yet
- Unit 5 EstándarDocument2 pagesUnit 5 EstándardechillbroNo ratings yet
- IEC61508 GuideDocument11 pagesIEC61508 Guidesrbehera1987No ratings yet
- RTR Piping Inspection GuideDocument17 pagesRTR Piping Inspection GuideFlorante NoblezaNo ratings yet
- Rolls-Royce M250 FIRST Network: 2015 Customer Support DirectoryDocument76 pagesRolls-Royce M250 FIRST Network: 2015 Customer Support Directoryale11vigarNo ratings yet
- STPM Chemistry Topic 17 Hydroxyl Compound (Short Notes)Document1 pageSTPM Chemistry Topic 17 Hydroxyl Compound (Short Notes)Chris Lau100% (1)
- Distinction Tests 12th Shobhit NirwanDocument1 pageDistinction Tests 12th Shobhit NirwanAkshaj TiwariNo ratings yet
- Distinction Tests 12th Shobhit NirwanDocument1 pageDistinction Tests 12th Shobhit NirwandivyanshaNo ratings yet
- Distinction Tests 12thDocument1 pageDistinction Tests 12thmadhavsingh9aNo ratings yet
- Distinction Tests 12thDocument1 pageDistinction Tests 12thRekha RaniNo ratings yet
- Distinction Tests 12th Shobhit NirwanDocument1 pageDistinction Tests 12th Shobhit Nirwankaustubhkushagra9No ratings yet
- Distinction Tests 12th Shobhit NirwanDocument1 pageDistinction Tests 12th Shobhit NirwanArush DhawalNo ratings yet
- Distinction Tests 12th Shobhit NirwanDocument1 pageDistinction Tests 12th Shobhit NirwanToxicVFXNo ratings yet
- Distinction Tests 12th Shobhit NirwanDocument1 pageDistinction Tests 12th Shobhit NirwanItz For YouNo ratings yet
- Distinction Tests 12th Shobhit NirwanDocument1 pageDistinction Tests 12th Shobhit Nirwanrishabhkushwaha3007No ratings yet
- Distinction Tests 12th Shobhit NirwanDocument1 pageDistinction Tests 12th Shobhit Nirwanaarushitv.11No ratings yet
- Distinction Tests 12th Shobhit NirwanDocument1 pageDistinction Tests 12th Shobhit NirwansharmashraneyNo ratings yet
- Distinction Tests 12th Shobhit NirwanDocument1 pageDistinction Tests 12th Shobhit NirwankhushiibhojakNo ratings yet
- Distinction Tests 12th Shobhit Nirwan - 231124 - 035956Document1 pageDistinction Tests 12th Shobhit Nirwan - 231124 - 035956mahatosandip1888No ratings yet
- Distinction Tests 12thDocument1 pageDistinction Tests 12thRishit JainNo ratings yet
- Distictions CBSE - Quick Revision NotesDocument2 pagesDistictions CBSE - Quick Revision NotesAdithya ShibuNo ratings yet
- Table-All Distinguish TestDocument1 pageTable-All Distinguish TestSubhranshu Sekhar DashNo ratings yet
- Chemical Test To Distinguish Between Pair of Organic CompoundDocument11 pagesChemical Test To Distinguish Between Pair of Organic CompoundHishq Dhiman100% (1)
- Organic functional group testsDocument19 pagesOrganic functional group testsThị Thu Trang NguyễnNo ratings yet
- Chemical Test Orgnic Chemistry 2020Document4 pagesChemical Test Orgnic Chemistry 2020Mukesh GanjawalaNo ratings yet
- Prelims HacksDocument1 pagePrelims HacksBEA FRANCINE DELOS SANTOSNo ratings yet
- Lec-01_Principles Related to Practical Organic Chemistry (Functional Group)_YN501MA_English_Sonal Madad_Krishna KumarDocument33 pagesLec-01_Principles Related to Practical Organic Chemistry (Functional Group)_YN501MA_English_Sonal Madad_Krishna KumarpriyadarshiNo ratings yet
- Aldehydes and KetonesDocument24 pagesAldehydes and KetonesMichael Angelo FilomenoNo ratings yet
- Qualitative Analysis Notes: Cedar College Salt Analysis Theory 1Document19 pagesQualitative Analysis Notes: Cedar College Salt Analysis Theory 1Daniyal KhanNo ratings yet
- Detection of Organic Functional GroupDocument1 pageDetection of Organic Functional Groupchandan3biswasNo ratings yet
- Chem 12 Organic DistinguishDocument5 pagesChem 12 Organic DistinguishNabaratna Biswal0% (1)
- CBSE Class 12 Chemistry Chemical Test To Distinguish Between Pair of Compounds - 0Document4 pagesCBSE Class 12 Chemistry Chemical Test To Distinguish Between Pair of Compounds - 0Ritu Raj100% (8)
- BARIUM CHLORIDE Ex. 11Document6 pagesBARIUM CHLORIDE Ex. 11wizard hamdsNo ratings yet
- Test Observation Conclusion: The City School, Ravi Campus (Johar Town Lahore) O Level ChemistryDocument3 pagesTest Observation Conclusion: The City School, Ravi Campus (Johar Town Lahore) O Level ChemistryTayyabaNo ratings yet
- Practical Chemistry-Theroy & Excercise Module-6-4Document58 pagesPractical Chemistry-Theroy & Excercise Module-6-4Raju SinghNo ratings yet
- Selected Reactions of Some AnionsDocument5 pagesSelected Reactions of Some AnionsJay JayNo ratings yet
- Complete Organic Chemistry (Brahmastra) Part 2Document763 pagesComplete Organic Chemistry (Brahmastra) Part 2mohdamaankhan74No ratings yet
- Type Method Determination Of: Condition Titrant Primary STD Indicator Observable Change and ReactionsDocument3 pagesType Method Determination Of: Condition Titrant Primary STD Indicator Observable Change and ReactionsbiotechNo ratings yet
- Distiguishing Tests For Pairs of Organic CompoundsDocument7 pagesDistiguishing Tests For Pairs of Organic CompoundsParam SoniNo ratings yet
- Tests for functional groups in organic compoundsDocument4 pagesTests for functional groups in organic compoundsRudraksh mittalNo ratings yet
- TestDocument52 pagesTestShivam Mittal100% (1)
- Qualitative inorganic analysis cation identificationDocument6 pagesQualitative inorganic analysis cation identificationgabby fosterNo ratings yet
- Test Reagents Identification ChartDocument2 pagesTest Reagents Identification ChartdgfdgsdfgsdsdgNo ratings yet
- Properties and Reactions of Alkanes (38 charactersDocument3 pagesProperties and Reactions of Alkanes (38 charactersidon'tgiveachogiwaNo ratings yet
- ၁၀တန်းOrganic chemistry summaryDocument6 pages၁၀တန်းOrganic chemistry summarySANLU HTUTNo ratings yet
- Lecture1 All About AnionDocument20 pagesLecture1 All About AnionAlma PustaNo ratings yet
- Chemistry Form 6 Sem 3 10Document29 pagesChemistry Form 6 Sem 3 10Anonymous WAnr0jvNo ratings yet
- Ultima HydrocarbonDocument124 pagesUltima HydrocarbonKrish RawatNo ratings yet
- Organic Functional Group Tests - дубль2Document2 pagesOrganic Functional Group Tests - дубль2Alina SuminaNo ratings yet
- Lab Expts 3 To 4 ReviewDocument4 pagesLab Expts 3 To 4 ReviewKyra Bianca R. FamacionNo ratings yet
- Zoom International School: Analysis of Acidic Radicals Test For Sulphate (SO) Ion Experiment Observation Inference SODocument6 pagesZoom International School: Analysis of Acidic Radicals Test For Sulphate (SO) Ion Experiment Observation Inference SOSiddhant SinghNo ratings yet
- RADHA SWAMI ACADEMY: Aldehydes, Ketones and Carboxylic AcidsDocument10 pagesRADHA SWAMI ACADEMY: Aldehydes, Ketones and Carboxylic AcidsVishal KushwahNo ratings yet
- Order of Experiments: Color Solubility Experiment Result SaltDocument4 pagesOrder of Experiments: Color Solubility Experiment Result SaltEshwar Parthiban100% (1)
- Group III Cation Analysis and Reagent PreparationDocument3 pagesGroup III Cation Analysis and Reagent PreparationLloyd EscanillaNo ratings yet
- N-Butyl Alcohol (1 Sec-Butyl Alcohol (2 Tert-Butyl Alcohol (3Document2 pagesN-Butyl Alcohol (1 Sec-Butyl Alcohol (2 Tert-Butyl Alcohol (3Alyssa CubillaNo ratings yet
- Distinguish Organic Compounds with Single Chemical TestsDocument1 pageDistinguish Organic Compounds with Single Chemical TestsChetan KumarNo ratings yet
- Aldehydes and KetonesDocument12 pagesAldehydes and KetonesSalwa KaramanNo ratings yet
- CP 07 & CP 15 - Analysis of Unknown CompoundsDocument5 pagesCP 07 & CP 15 - Analysis of Unknown Compoundsdameesh9No ratings yet
- SPM Chemistry Formula List Form5 PDFDocument15 pagesSPM Chemistry Formula List Form5 PDFshuyiNo ratings yet
- Chemistry Concept MapDocument55 pagesChemistry Concept MapKaia GuarteNo ratings yet
- Distinction Between Organic Compounds Chemical TestDocument8 pagesDistinction Between Organic Compounds Chemical TestMission Security Services Pvt. LtdNo ratings yet
- Section IIIDocument3 pagesSection IIIbabypowder4No ratings yet
- Graphene-based Carbocatalysis: Synthesis, Properties and Applications: Volume 1From EverandGraphene-based Carbocatalysis: Synthesis, Properties and Applications: Volume 1No ratings yet
- JMPRTraininga I5545e PDFDocument500 pagesJMPRTraininga I5545e PDFmvptoxNo ratings yet
- Introduction To Financial Planning Unit 1Document57 pagesIntroduction To Financial Planning Unit 1Joshua GeddamNo ratings yet
- Community HelpersDocument3 pagesCommunity Helpersapi-252790280100% (1)
- Lecture Euler EquationDocument33 pagesLecture Euler EquationYash RajNo ratings yet
- Concise Operating Instructions: Frequency Converter For HOISTING - TRAVEL (Siemens)Document9 pagesConcise Operating Instructions: Frequency Converter For HOISTING - TRAVEL (Siemens)Pablo Hidalgo ValenzuelaNo ratings yet
- Inner Unit EstimateDocument35 pagesInner Unit EstimateMir MoNo ratings yet
- DataSheet IMA18-10BE1ZC0K 6041793 enDocument8 pagesDataSheet IMA18-10BE1ZC0K 6041793 enRuben Hernandez TrejoNo ratings yet
- Bài tập tiếng Anh 12 (Reading)Document7 pagesBài tập tiếng Anh 12 (Reading)Minh AnhNo ratings yet
- Intermediate Accounting 2 - CL NCL Lecture NotesDocument2 pagesIntermediate Accounting 2 - CL NCL Lecture NotesRacheel SollezaNo ratings yet
- 3343 - C-Data-EPON-OLT-FD1108S-CLI-User-Manual-V1-3Document82 pages3343 - C-Data-EPON-OLT-FD1108S-CLI-User-Manual-V1-3Roar ZoneNo ratings yet
- If Sentences Type 1 First Type Conditionals Grammar Drills - 119169Document2 pagesIf Sentences Type 1 First Type Conditionals Grammar Drills - 119169Ivanciu DanNo ratings yet
- Rg213 Rgflex Coax Braided Cable: Product Data Sheet RG213-50JFDocument1 pageRg213 Rgflex Coax Braided Cable: Product Data Sheet RG213-50JFPancho BerríosNo ratings yet
- Computer ViruesDocument19 pagesComputer ViruesMuhammad Adeel AnsariNo ratings yet
- Annual Report 18Document363 pagesAnnual Report 18Safeer UllahNo ratings yet
- School of Education, Arts and Sciences General Education Area 1 SEMESTER S.Y 2021-2022Document4 pagesSchool of Education, Arts and Sciences General Education Area 1 SEMESTER S.Y 2021-2022JaylordPalattaoNo ratings yet
- GSAA HET 2005-15, Tranche B2 / BSABS 2005-TC2, Tranche M6 Shown As An Asset of Maiden LaneDocument122 pagesGSAA HET 2005-15, Tranche B2 / BSABS 2005-TC2, Tranche M6 Shown As An Asset of Maiden LaneTim BryantNo ratings yet
- Automatic Repeat Request (Arq)Document15 pagesAutomatic Repeat Request (Arq)Rahul RedkarNo ratings yet
- From Memphis To KingstonDocument19 pagesFrom Memphis To KingstonCarlos QuirogaNo ratings yet
- Atlas Ci30002Tier-PropanDocument3 pagesAtlas Ci30002Tier-PropanMarkus JeremiaNo ratings yet
- 4) April 2023 Current AffairsDocument24 pages4) April 2023 Current AffairsPicturesque vibrant shadesNo ratings yet
- Atomic Structure QuestionsDocument1 pageAtomic Structure QuestionsJames MungallNo ratings yet
- Covid 19 PDFDocument117 pagesCovid 19 PDFvicky anandNo ratings yet
- CS6711 Security Lab ManualDocument84 pagesCS6711 Security Lab ManualGanesh KumarNo ratings yet
- Nettoplcsim S7online Documentation en v0.9.1Document5 pagesNettoplcsim S7online Documentation en v0.9.1SyariefNo ratings yet