Professional Documents
Culture Documents
How Boiling Point Varies With Vapor Pressure - Antonie Equation - Pharma Engineering
Uploaded by
Rana krupalOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
How Boiling Point Varies With Vapor Pressure - Antonie Equation - Pharma Engineering
Uploaded by
Rana krupalCopyright:
Available Formats
10/19/23, 12:43 PM How Boiling point varies with Vapor Pressure - Antonie Equation - Pharma Engineering
Calculations Contact Me Online
TheTraining Calculators would
website “Engineering” Downloads
like to Equipment Design How To Softwares Process Safety About Me
send you push notifications.
Pharma Engineering
Notifications can be turned off anytime from browser
settings.
MSC tailors for you complete shipping solutions SIGN U
for engineer by engineer Powered by Don't Allow Allow from the field to the final destination
Home antonie equation boiling point Calculations vapour pressure How Boiling point varies with Vapor Pressure - Types of Agitators, Agitato
Search
Antonie Equation Significance
How Boiling point varies with Vapor Pressure -
[HOW TO] Calculate Densi
Antonie Equation Mixture
Ajay Kumar 21:57:00 antonie equation, boiling point, Calculations, vapour pressure
Tonne of Refrigeration, Ho
required TR
[How To] Select a Motor C
Agitator
[How To] Design a Scrubb
column ] (UPDATED) as on
[How To] Calculate Energy
[HOW TO]Calculate the Vo
by Torispherical Dish
Overall Heat Transfer Co-E
Calculation
[How to] find Reactor Hea
Theoretically
Agitated Thin Film Dryer (
Hey visitors......!!! & Design Calculations
The most basic thing that many engineers were aware of that is Boiling point varies with Vapour pressure, and i'll bet you that many of
Leave
those Engineers donno the exact reason for this, and today i'll reveal that under hidden reason a message
for that behavior of solvents. Bsic SixSigma Metrics
1 Hello everyone,Today i'm going to s
... read more
For that you should be aware of some damn basic things like what does exactly Vapour pressure, Boiling point means. May 26 2023
Estimation of orifice size for dosing
What is Vapour Pressure ? 2 Good morning everyone....!!!Hope e
safe ... read more
May 03 2023
Vapour Pressure means the pressure exerted by the vapour on the surface of liquid at equilibrium, Usually vapour wont have any
Process Capability & Performance
Vapour pressures, the vapour pressure is the property of solvents, every solvent will have their respective vapour pressures.
3 Cpk, Pp, Ppk)
Hello Everyone ....!!!Hope everythin
What is Boiling Point ?
read more
Apr 03 2023
Stossels Criticality Index - Classific
Boiling point is nothing but a point of saturation where the vapour pressure of a solvent equals the atmospheric pressure, simply
4 reactions
toluene is a solvent, whose boiling point is 110.6°C, and at that boiling point the vapour pressure of toluene will be 760 torr, [Dont get Good day everyone ......!!Hope every
read more
confused Torr means mmHg only]. And same is the case with every solvents boiling point.
Apr 01 2023
[How To] Preparation of a Raw Ma
Also Read:
5 Contribution (RMCC) Sheet for a
Pharmaceutical Product
Below is our detailed video on prepa
read more
Mar 21 2023
https://www.pharmacalculations.com/2016/12/how-boiling-point-varies-with-vapor.html#:~:text=the Vacuum should be NLT,760 - 650 %3D 110 m… 1/7
10/19/23, 12:43 PM How Boiling point varies with Vapor Pressure - Antonie Equation - Pharma Engineering
[How To] Prepare a Capacity shee
How to Select a Condenser? The website “Engineering” would like to
6 pharmaceutical product
send you push notifications.
Below is our detailed video on prepa
Notifications can be turned off anytime from browser
What Does a TR exactly means? read more
settings. Mar 19 2023
How to Calculate the Energy
Powered by of Steam? Estimation of Liquid Nitrogen cons
7 Hello everyone....!!First of all, Happ
read more
Mar 08 2023
Right now, you got a clear idea of the Vapour Pressure & Boiling point, So now i'll start my show about describing the relation ship Estimation of Oxygen Depletion
8 Good morning Everyone ...!!I've be
between Vapour Pressure and Boiling point, the only relation that describes the relation between these two parameters is Antonie
... read more
Equation. Sep 11 2022
Fire Load Calculation & Estimating
By using Antonie equation we can calculate the boiling point of solvent at different pressures, it also means that the boiling point of 9 Extinguishers Requirement
solvents will decrease with Vacuum, and we can calculate it theoretically. In fact, this is the first calculation that i've learned after
Hello Everyone, Hope everything is
Jun 26 2022
joining the Pharma Industry.
Estimating Booster Pump Requirem
10 Vacuum System
The basic Antonie Equation is, Img Credits: A&J Vacuum ServicesG
read more
Jun 12 2022
Log P = A - B / ( T + C ),
Type Here...
A, B, C are Antonie Constants,
DONATE HERE
P is Vapour Pressure, T is Boiling point.
Just remember one thing, while selecting the Antonie constants, there are many set of constants available, and they will vary with the Training on Process Engin
units of Pressure that you choose.
So, by now, if you are new to this calculation, this known equation will look something special to you,
Recommended Posts For You:
How to Scale Up?
Industrial Column design steps.........!!
How to calculate time cycle of Batch reactor distillation?
Leave a message
Read Post in your languag
Many of the Pharma Operations will include "Distill off solvent under vacuum below temperature X°C" , here while they include word
Select Language Powered by
vacuum just because we can distill off the solvent below its regular boiling point.
I'll show you a small demo and Anyway i'll tell you how to use this practically, Join Our Newslet
"Distill off toluene under vacuum below 60°C with vacuum NLT 650 mmHg at the end of distillation",
Discover related topics
Get All The Latest Updates De
Can Water Flow in Vacuum Straight Into Your Inbox For
Different Characterization Between Compressed Liquid and Superheated Vapor ENTER YOUR EMAIL ADDRES
JOIN NOW
Vapor Pressure in Open Container
Water Has a Heat of Vaporization of 44.01 Kj Mol
Followers
Metal Melting Point in Vacuum
https://www.pharmacalculations.com/2016/12/how-boiling-point-varies-with-vapor.html#:~:text=the Vacuum should be NLT,760 - 650 %3D 110 m… 2/7
10/19/23, 12:43 PM How Boiling point varies with Vapor Pressure - Antonie Equation - Pharma Engineering
Followers (64) Next
The website “Engineering” would like to
send you push notifications.
Notifications
This means the product that is present in our reactioncan be is
mass turned
stableoffupto
anytime from
60°C, browser
and after that the product may degrade,
settings.
Powered by
And here vacuum should be NLT 650 mmHg at the end of distillation, so now just try to calculate the boiling point at 650 mmHg,
Follow
For that you need to know the Antonie constants of Toluene,
Antonie constants data here
A = 6.95, B = 1344.8, C = 219.482,
the Vacuum should be NLT 650 mmHg, so the pressure should be P = 760 - 650 = 110 mmHg.
Log (110 ) = 6.95 - 1344.8 / ( T + 219.482 )
Solving for T, T = 54.4°C. Pharma Engineering need auth
Join me and publish your stuff o
So, this means at 650 mmHg the boiling point of Toluene will be 54.4°C, which means it came down from 110.6°C to 54.4°C.
No charges for publishing
So, if you understand what i delivered above, just say cheers,
Still any doubts feel free to contact me,
Comments were most appreciated...............!!!
Also Read:
How to Design a Condenser?
How to Select a Pump , Motor, Line Sizings ?
Blog Archive
► 2023 (7)
► 2022 (4)
How To Select a Condenser?
Leave a message ► 2021 (2)
► 2020 (27)
Determine the Power Required for an Operation? ► 2019 (11)
► 2018 (13)
Industrial Distillation Column Design Steps ► 2017 (11)
▼ 2016 (32)
▼ December (1)
How Boiling point varies with Vap
Ant...
About The Author ► November (1)
► October (3)
► September (1)
► June (5)
Hi! I am Ajay Kumar Kalva, Currently serving as the CEO of this site, a tec
► May (10)
geek by passion, and a chemical process engineer by profession, i'm intereste ► April (6)
in writing articles regarding technology, hacking and pharma technology. ► March (4)
► February (1)
Follow Me on Twitter AjaySpectator & Computer Innovations
► 2015 (5)
Your Ads Here
Share This: Facebook Twitter Google+ Pinterest Linkedin
vapour pressure
17 comments:
https://www.pharmacalculations.com/2016/12/how-boiling-point-varies-with-vapor.html#:~:text=the Vacuum should be NLT,760 - 650 %3D 110 m… 3/7
10/19/23, 12:43 PM How Boiling point varies with Vapor Pressure - Antonie Equation - Pharma Engineering
ANSHU BHARGAVA 3 JANUARYThe
2017website
AT 12:21 “Engineering” would like to
send you push notifications.
good.
Notifications can be turned off anytime from browser
Reply settings.
Powered by
KANDIPALLI SATISH 8 FEBRUARY 2017 AT 22:22
Good
Reply
Replies
1. UNKNOWN 29 MARCH 2017 AT 18:00
how you calculated T value?
2. AJAY KUMAR AUTHOR 30 MARCH 2017 AT 06:27
if you know the vapour pressure just insert in Antonie equation, and solve for T, that's it....!!!
Regards,
PHARMA ENGINEERING
Reply
RAVI KUMAR 10 NOVEMBER 2017 AT 10:15
thanks for ur valuable post.
Reply
UNKNOWN 13 NOVEMBER 2017 AT 18:45
Dear Ajay,
For selection of P value, do we have to substract required Vacuum (i.e.,650mmHg in case you have considered) from
760 mmHg or the atmospheric pressure at the given location (for example ~720mmHg in Hyderabad)??
Thanks
Reply
Replies
1. AJAY KUMAR AUTHOR 13 NOVEMBER 2017 AT 21:27
Dear Khan,
correct, you need to subtract from atnospheric pressure, i.e., 760 mmHg.
Leave a message
Regards,
AJAY K
2. UNKNOWN 13 NOVEMBER 2017 AT 21:35
Atmospheric pressure varies with sea level.Then, how do we consider that?
3. AJAY KUMAR AUTHOR 14 NOVEMBER 2017 AT 06:08
Dear Khan,
please go through the post, and try to calculate the atmospheric pressure at different
locations based on height of sea level. Casually i've mentioned atmospheric pressure i.e.,
760mmHg.
Regards,
AJAY K
Reply
KEDAR 29 APRIL 2019 AT 22:11
Hello this is kedar,
How you find T value is 54.4,
https://www.pharmacalculations.com/2016/12/how-boiling-point-varies-with-vapor.html#:~:text=the Vacuum should be NLT,760 - 650 %3D 110 m… 4/7
10/19/23, 12:43 PM How Boiling point varies with Vapor Pressure - Antonie Equation - Pharma Engineering
By calculating the equation T value comes minus 874.96
The website “Engineering” would like to
Pls varifie the euation i;e log (110)=(6.95-1344.8)/(T+219.482)
Pls check. send you push notifications.
Notifications can be turned off anytime from browser
Reply
settings.
Powered by
KEDAR 29 APRIL 2019 AT 22:16
Hello this is kedar,
How you calculate log110= (6.95-1344.8)/(T+219.482)
Its comes in minus value ,
But not 54.4
Pls check & reply.
Reply
Replies
1. AJAY KUMAR AUTHOR 30 APRIL 2019 AT 05:59
Hii Kedar,
You read the equation wrong, its Log(110) = 6.95 - (1344.8/(T+219.482)).
Now once try repeating the calculation, you will get it.
Best Regards,
AJAY K
Reply
KEDAR 1 MAY 2019 AT 09:19
Hello this is kedar,
Antonie constant are always same, if we take pressure unit is mmhg then its value is constant ? If we take pressure
unit is kg/cm2 then whats it value ?pls reply.
Reply
Replies
1. AJAY KUMAR AUTHOR 1 MAY 2019 AT 12:33
Hii Kedar,
The constants will surely vary based on the units of pressure and temperature,
As we are much familiar with mmHg and C, i've given those.
Regards,
AJAY K
Reply
Leave a message
UNKNOWN 25 SEPTEMBER 2019 AT 22:38
Hi
This Rachit
How we find out the values of A,B,C for different types of solvents
please suggest.
Reply
Replies
1. AJAY KUMAR AUTHOR 27 SEPTEMBER 2019 AT 16:02
Hii Rachit,
Pl click on the link provided, 'ANTONIE CONSTANTS DATA HERE'.
Regards.
Reply
UNKNOWN 1 JUNE 2020 AT 17:33
https://www.pharmacalculations.com/2016/12/how-boiling-point-varies-with-vapor.html#:~:text=the Vacuum should be NLT,760 - 650 %3D 110 m… 5/7
10/19/23, 12:43 PM How Boiling point varies with Vapor Pressure - Antonie Equation - Pharma Engineering
Hi,
The website
i am not able to access the antonies “Engineering”
constants data here link would like to
please help
Thank you send you push notifications.
Notifications can be turned off anytime from browser
Reply
settings.
Powered by
Enter comment
Newer Post Older Post
Leave a message
https://www.pharmacalculations.com/2016/12/how-boiling-point-varies-with-vapor.html#:~:text=the Vacuum should be NLT,760 - 650 %3D 110 m… 6/7
10/19/23, 12:43 PM How Boiling point varies with Vapor Pressure - Antonie Equation - Pharma Engineering
The website “Engineering” would like to
send you push notifications. ABOUT ADMIN Labels
Notifications can be turned off anytime from browser
agitator Calculations chilling Condense
settings. Hi! I am Ajay Kumar Kalva, owner of this
site, a tech geek by passion, and a design Documentation DQ FTA how
Powered by
chemical process engineer by
profession, i'm interested in writing iq network analysis OOS OQ
articles regarding technology, hacking and pharma
Power consumption pressure calculation p
technology.
reaction kinetics scaleups simulation
timecycle for heating/cooling Tons of Refrigerat
Like Us On Facebook
utilities vacuum pump
This Blog is protected by DMCA.com
Get our App on Play Store
playstore
© 2015 - 2023 All Rights Reserved. PharmaCalculations.com || Created By Computer Innovations | Designed & Customised by Ajay Kumar Kalva
Leave a message
https://www.pharmacalculations.com/2016/12/how-boiling-point-varies-with-vapor.html#:~:text=the Vacuum should be NLT,760 - 650 %3D 110 m… 7/7
You might also like
- Pipe Supports GuideDocument32 pagesPipe Supports GuideMANOEL JUNIORNo ratings yet
- Types of Agitators, Agitator's Design and Significance - Pharma EngineeringDocument23 pagesTypes of Agitators, Agitator's Design and Significance - Pharma EngineeringMuhammad Erwin Yamashita33% (3)
- Butyraldehyde Final ReportDocument85 pagesButyraldehyde Final ReportRana krupal100% (1)
- Design A Scrubber (Packed Column)Document14 pagesDesign A Scrubber (Packed Column)Manali PatilNo ratings yet
- Types of Agitators, Agitator's Design and Significance - Pharma EngineeringDocument58 pagesTypes of Agitators, Agitator's Design and Significance - Pharma Engineeringmyself_riteshNo ratings yet
- An Introduction To Thermal Physics - 1Document237 pagesAn Introduction To Thermal Physics - 1PranayYadav100% (3)
- A320-Engine Failure (No Damage)Document13 pagesA320-Engine Failure (No Damage)tugayyoung100% (1)
- Me 6604 GDJP Notes Five UnitsDocument146 pagesMe 6604 GDJP Notes Five UnitsAnonymous MfH5TN2anNo ratings yet
- Lecture 1 Vacuum Systems - V Baglin - JUAS 2017 - 14 Feb 2017Document76 pagesLecture 1 Vacuum Systems - V Baglin - JUAS 2017 - 14 Feb 2017Lê Văn Thế QuangNo ratings yet
- Complette Course On RefrigerationDocument746 pagesComplette Course On RefrigerationVlad MartianNo ratings yet
- Gas Piping SizeDocument1 pageGas Piping Sizespmg222No ratings yet
- Bernoulli's Theorem ExperimentDocument17 pagesBernoulli's Theorem ExperimentMimi Hashim95% (19)
- Report Update PT. PIS 20 Desember 2019Document2 pagesReport Update PT. PIS 20 Desember 2019Tika LorenzaNo ratings yet
- (How To) Select A Motor Capacity For Agitator - Pharma EngineeringDocument9 pages(How To) Select A Motor Capacity For Agitator - Pharma EngineeringpratikNo ratings yet
- How To Calculate Wheel Torque From Engine TorqueDocument7 pagesHow To Calculate Wheel Torque From Engine TorqueDevakumarNo ratings yet
- Political Compass V 0.1. 2016: Alejandro Zaera-Polo & Guillermo Fernandez-AbascalDocument5 pagesPolitical Compass V 0.1. 2016: Alejandro Zaera-Polo & Guillermo Fernandez-AbascalAna CristeaNo ratings yet
- Pharma Engineering: (How To) Calculate NPSH - Net Positive Suction HeadDocument8 pagesPharma Engineering: (How To) Calculate NPSH - Net Positive Suction HeadpratikNo ratings yet
- (How To) Calculate The Required Blower Capacity For FBD - Pharma EngineeringDocument11 pages(How To) Calculate The Required Blower Capacity For FBD - Pharma EngineeringTerrence Terry BhengoeNo ratings yet
- Submit SOFL forms for plant performance feedbackDocument5 pagesSubmit SOFL forms for plant performance feedbackSujay Hazra100% (1)
- Sensitivity: LNT Construction Internal UseDocument5 pagesSensitivity: LNT Construction Internal UseAnjum JauherNo ratings yet
- HOW TO - The And, Or, XOR Logic Operations On CONTACTs Inputs LDmicro - LDmicro Wiki GitHubDocument1 pageHOW TO - The And, Or, XOR Logic Operations On CONTACTs Inputs LDmicro - LDmicro Wiki GitHubvanjalujicNo ratings yet
- R01 - Production LogDocument2 pagesR01 - Production LogManigandan RNo ratings yet
- Flow process Biologi PT SHARPDocument1 pageFlow process Biologi PT SHARPagus purwantoNo ratings yet
- List of Material Submittals Action Submittal Review EstimateDocument6 pagesList of Material Submittals Action Submittal Review EstimatejerconsNo ratings yet
- Form XVII Register Of WagesDocument3 pagesForm XVII Register Of WagesBalakrishna HNo ratings yet
- Record of Single Machine (II) Centrifugal Compressors: SH 3503-J314-2CDocument4 pagesRecord of Single Machine (II) Centrifugal Compressors: SH 3503-J314-2CEason HuangNo ratings yet
- Formato e (L-E 2.14)Document1 pageFormato e (L-E 2.14)Jorge Luis Romero díazNo ratings yet
- Maintenance Central Workshop Result Inspection ElectricalDocument6 pagesMaintenance Central Workshop Result Inspection ElectricalMuhammad ArifinNo ratings yet
- Software Requisition - Form - NitinDocument4 pagesSoftware Requisition - Form - NitinlavakerreddyNo ratings yet
- Sample LayoutDocument1 pageSample LayoutMuhammad Ali KhanNo ratings yet
- In-House Worker: Total Hrs Total Hrs Total Hrs Total Hrs Total Hrs Total HrsDocument1 pageIn-House Worker: Total Hrs Total Hrs Total Hrs Total Hrs Total Hrs Total HrsGenrey ConstructionNo ratings yet
- Mwathi-Utility-Bill-1-Pdf-Free - 1 - 1Document1 pageMwathi-Utility-Bill-1-Pdf-Free - 1 - 1afrimall.storeNo ratings yet
- P2TL 14 CDocument12 pagesP2TL 14 CArum YunitaNo ratings yet
- Adobe Scan 07-Jun-2023Document1 pageAdobe Scan 07-Jun-2023Hardik JindalNo ratings yet
- Flywheel Effect or Polar Moment of Inertia - Engineers EdgeDocument2 pagesFlywheel Effect or Polar Moment of Inertia - Engineers Edgestallone21No ratings yet
- Chemical & Process Technology: Pressure VesselsDocument4 pagesChemical & Process Technology: Pressure VesselsAmlan SahaNo ratings yet
- LKCDocument4 pagesLKCMatheus BuenoNo ratings yet
- Cerramiento FC Plaza Center RexDocument1 pageCerramiento FC Plaza Center Rexangie castañeda millaNo ratings yet
- 02 DrawingDocument1 page02 DrawingAbm Faruk E MonjurNo ratings yet
- Power Plant and Calculations - Calculation of Coal Handling Plant and Bunker CapacityDocument7 pagesPower Plant and Calculations - Calculation of Coal Handling Plant and Bunker CapacityRajeshNo ratings yet
- Daily construction progress reportDocument2 pagesDaily construction progress reportSyed Adnan AqibNo ratings yet
- DPRDocument2 pagesDPRSyed Adnan AqibNo ratings yet
- 1.1 Air Conditioing and Mechanical Ventilation System: Hvac System Calculation SheetDocument1 page1.1 Air Conditioing and Mechanical Ventilation System: Hvac System Calculation Sheetefmartin21No ratings yet
- BETAGRO GY ES 001 Rev 01 DC Voltage Drop and Cable Sizing CalculationDocument5 pagesBETAGRO GY ES 001 Rev 01 DC Voltage Drop and Cable Sizing CalculationThai ChheanghourtNo ratings yet
- Devansh Chouksey, Acc W Tally, Q1Document5 pagesDevansh Chouksey, Acc W Tally, Q1Sangeeta YadavNo ratings yet
- Pump Sizing Calculation - Twopump RunningDocument2 pagesPump Sizing Calculation - Twopump RunningMuthuKumar ArunachalamNo ratings yet
- SayfaDocument1 pageSayfadoğancanNo ratings yet
- Job Card: A1 MotorsDocument2 pagesJob Card: A1 MotorsSingamaneni Srinivasa RaoNo ratings yet
- SJ71-00055D (08Q) Cylinder CAN Process Inspection Check Sheet (SE)Document6 pagesSJ71-00055D (08Q) Cylinder CAN Process Inspection Check Sheet (SE)segar PalanisamyNo ratings yet
- FJC KP 00R 11 Jan 23Document1 pageFJC KP 00R 11 Jan 23Aulia AchmadNo ratings yet
- Red1 Compiere Workshop: Mix-MatchDocument9 pagesRed1 Compiere Workshop: Mix-MatchWilson GayoNo ratings yet
- Eh Ad 2.eheh Iac 03 3Document1 pageEh Ad 2.eheh Iac 03 3patrouilledeafranceNo ratings yet
- 03.WHM List 20230426Document1 page03.WHM List 20230426luvinhdieuNo ratings yet
- CONVEYORDocument1 pageCONVEYORfaiqnashrullahNo ratings yet
- Sonda GenesisDocument2 pagesSonda GenesisSergio Cordova SozaNo ratings yet
- Februarie 2022 cheltuieli pentru asociatia de proprietari Snagov Nr. 2BDocument1 pageFebruarie 2022 cheltuieli pentru asociatia de proprietari Snagov Nr. 2BRazvanHaragNo ratings yet
- A2 Plant Operation Log Book CPGL - New (Final)Document1 pageA2 Plant Operation Log Book CPGL - New (Final)Forida EasminNo ratings yet
- FJC KP 00R 21 DesDocument1 pageFJC KP 00R 21 DesAulia AchmadNo ratings yet
- Type - A Manhole BBS FileDocument1 pageType - A Manhole BBS FilefbdfNo ratings yet
- Fees - and - Checklist Page 2Document1 pageFees - and - Checklist Page 2jumbabsNo ratings yet
- Dashboard DPR BBP 07 Desember 2023Document1 pageDashboard DPR BBP 07 Desember 2023vorda buaymadangNo ratings yet
- Mallikarjuna RL 14 5675Document1 pageMallikarjuna RL 14 5675Engineering Assistant GangavaramNo ratings yet
- 05 Euro4 SoftwereDocument6 pages05 Euro4 SoftwereSergeyNo ratings yet
- ANEVAZO Plant Layout GuideDocument1 pageANEVAZO Plant Layout GuideAlyssa Sarah ArenasNo ratings yet
- ABB Basic Pressure Measurement WhitePaperDocument6 pagesABB Basic Pressure Measurement WhitePapermlaouhi MajedNo ratings yet
- Earth Overshoot Day 2023 Nowcast ReportDocument9 pagesEarth Overshoot Day 2023 Nowcast ReportRana krupalNo ratings yet
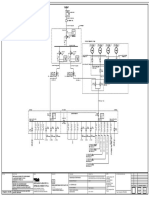
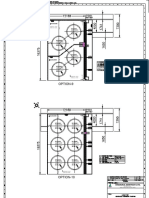
- Layout For Centralized Eme - Tankfarm - 20-02-24 - 5Document1 pageLayout For Centralized Eme - Tankfarm - 20-02-24 - 5Rana krupalNo ratings yet
- Jayant S Ullas - Praj IndustriesDocument1 pageJayant S Ullas - Praj IndustriesRana krupalNo ratings yet
- Layout For Centralized Eme - Tankfarm - 20-02-24 - 4Document1 pageLayout For Centralized Eme - Tankfarm - 20-02-24 - 4Rana krupalNo ratings yet
- Layout For Centralized Eme - Tankfarm - 20-02-24 - 3Document1 pageLayout For Centralized Eme - Tankfarm - 20-02-24 - 3Rana krupalNo ratings yet
- Carnot CycleDocument1 pageCarnot CycleRana krupalNo ratings yet
- IUPAC recommendations for organic chemistry nomenclatureDocument91 pagesIUPAC recommendations for organic chemistry nomenclatureSaurabh ShuklaNo ratings yet
- Layout For Centralized Eme - Tankfarm - 20-02-24 - 5Document1 pageLayout For Centralized Eme - Tankfarm - 20-02-24 - 5Rana krupalNo ratings yet
- Toluene MSDS summaryDocument8 pagesToluene MSDS summaryrafida aisyahNo ratings yet
- Safety Data Sheet: Tokyo Chemical Industry (India) Pvt. LTDDocument5 pagesSafety Data Sheet: Tokyo Chemical Industry (India) Pvt. LTDRana krupalNo ratings yet
- Rubber Chemical Resistance, Rubber Chemical Compatibility, Page 2Document12 pagesRubber Chemical Resistance, Rubber Chemical Compatibility, Page 2Quality ControlNo ratings yet
- 1.6! Drawing Chemical StructuresDocument6 pages1.6! Drawing Chemical StructuresSadeeq ArtxzNo ratings yet
- Aircon NotesDocument5 pagesAircon Notesprado01No ratings yet
- R-12 Table PDFDocument18 pagesR-12 Table PDFNaveen AgrawalNo ratings yet
- Ass - 1 (2021-22)Document3 pagesAss - 1 (2021-22)Nishith KumarNo ratings yet
- Diagram SketchingDocument3 pagesDiagram SketchingQuennie Marie AñanaNo ratings yet
- Review Physics 2: Date: Xx/Xx/20XxDocument21 pagesReview Physics 2: Date: Xx/Xx/20XxDương Hà Trúc TâmNo ratings yet
- Problem Set #2 WPS 760Document15 pagesProblem Set #2 WPS 760weilong9183% (12)
- States of Matter: Thermal EnergyDocument75 pagesStates of Matter: Thermal EnergyAtharv saxenaNo ratings yet
- CAIE Lesson Plan Template - ChemistryDocument5 pagesCAIE Lesson Plan Template - Chemistryvidya pmNo ratings yet
- Compressor Tech 2Document6 pagesCompressor Tech 2Elvis Alberto Rodriguez Bravo100% (1)
- Gas Absorption Process ExplainedDocument31 pagesGas Absorption Process Explainedamira nabillaNo ratings yet
- Line List with Piping Component Details for Plant 14Document29 pagesLine List with Piping Component Details for Plant 14rifaNo ratings yet
- Science ModDocument50 pagesScience ModJohary DisaloNo ratings yet
- Chapter 12. Heat Transfer To Fluids Without Phase ChangeDocument10 pagesChapter 12. Heat Transfer To Fluids Without Phase ChangeSwapna VadlamaniNo ratings yet
- Me 2204 - Fluid Mechanics and MachineryDocument3 pagesMe 2204 - Fluid Mechanics and MachineryKarthik SubramaniNo ratings yet
- Ternary Systems IIDocument29 pagesTernary Systems IIDivya SreeNo ratings yet
- Fluid Mechanics Course OverviewDocument2 pagesFluid Mechanics Course OverviewParamvir GuliaNo ratings yet
- Specific Heat and Phase Changes ExplainedDocument16 pagesSpecific Heat and Phase Changes ExplainedAli AkbarNo ratings yet
- MLL702-Assignment3Document3 pagesMLL702-Assignment3Kumar VibhamNo ratings yet
- Air Liquefaction - DistillationDocument16 pagesAir Liquefaction - DistillationElafanNo ratings yet
- Kandlikar - Heat Transfer During Flow BoilingDocument9 pagesKandlikar - Heat Transfer During Flow BoilingadrrineNo ratings yet
- Notes States of Matter & GassesDocument18 pagesNotes States of Matter & GassesKaran KapoorNo ratings yet
- Phase DiagramsDocument19 pagesPhase Diagramsget2csNo ratings yet
- TM.P. S.p.A. Pump Performance CurvesDocument1 pageTM.P. S.p.A. Pump Performance CurvesSiddiqui Muhammad AshfaqueNo ratings yet
- Batch and continuous distillation column typesDocument6 pagesBatch and continuous distillation column typesOmer IbrahimNo ratings yet