Professional Documents
Culture Documents
Trends Part 1
Trends Part 1
Uploaded by
Sahar Anjum0 ratings0% found this document useful (0 votes)
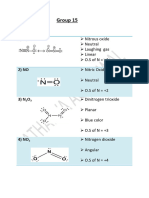
5 views1 pageThe document compares various properties of group 15 elements (nitrogen, phosphorus, arsenic, antimony, and bismuth) and their hydrides and oxides. It lists trends in atomic mass, ionization energy, electronegativity, covalent radius, ionic radius, melting point, and boiling point that generally increase down the group. It also compares properties of the group 15 hydrides like melting point, boiling point, E-H distance, H-E-H bond angle, acidic strength, and reducing character that tend to increase with increasing element size. Furthermore, it notes the acidic strength of the group 15 oxides increases from N2O3 to Bi2O3.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document compares various properties of group 15 elements (nitrogen, phosphorus, arsenic, antimony, and bismuth) and their hydrides and oxides. It lists trends in atomic mass, ionization energy, electronegativity, covalent radius, ionic radius, melting point, and boiling point that generally increase down the group. It also compares properties of the group 15 hydrides like melting point, boiling point, E-H distance, H-E-H bond angle, acidic strength, and reducing character that tend to increase with increasing element size. Furthermore, it notes the acidic strength of the group 15 oxides increases from N2O3 to Bi2O3.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
5 views1 pageTrends Part 1
Trends Part 1
Uploaded by
Sahar AnjumThe document compares various properties of group 15 elements (nitrogen, phosphorus, arsenic, antimony, and bismuth) and their hydrides and oxides. It lists trends in atomic mass, ionization energy, electronegativity, covalent radius, ionic radius, melting point, and boiling point that generally increase down the group. It also compares properties of the group 15 hydrides like melting point, boiling point, E-H distance, H-E-H bond angle, acidic strength, and reducing character that tend to increase with increasing element size. Furthermore, it notes the acidic strength of the group 15 oxides increases from N2O3 to Bi2O3.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
GROUP – 15
1) Atomic mass N < P < As < Sb < Bi
2) Ionisation enthalpy N > P > As > Sb > Bi
*3) Electronegativity N > P > As > Sb = Bi
4) Covalent radius N < P < As < Sb < Bi
*5) Ionic radius Sb < Bi < N < P < As
*6) Melting point N < P < Bi < Sb < As
*7) Boiling point N < P < As < Bi < Sb
8) Density N < P < As < Sb < Bi
GROUP 15 HYDRIDES
*1) Melting point PH3 < AsH3 < SbH3 < NH3
*2) Boiling Point PH3 < AsH3 < NH3 < SbH3 < BiH3
3) E-H distance (E = element) NH3 < PH3 < AsH3 < SbH3
4) HEH angle (E = element) NH3 > PH3 > AsH3 > SbH3
5) Acidic strength NH3 < PH3 < AsH3 < SbH3 < BiH3
6) Reducing character NH3 < PH3 < AsH3 < SbH3 < BiH3
GROUP 15 OXIDES
1) Acidic Strength N2O3 > P2O3 > As2O3 > Sb2O3 > Bi2O3
You might also like
- Materials Data for Cyclic Loading: Low-Alloy SteelsFrom EverandMaterials Data for Cyclic Loading: Low-Alloy SteelsRating: 5 out of 5 stars5/5 (2)
- Practice Problems - Gravimetric FactorsDocument2 pagesPractice Problems - Gravimetric FactorsElizaga ElizagaNo ratings yet
- Chemistry Reference TablesDocument8 pagesChemistry Reference Tablescauten2100% (1)
- TRENDS InorganicDocument9 pagesTRENDS InorganicscrbdddNo ratings yet
- Group - 16: Enthalpy of Dissociation)Document2 pagesGroup - 16: Enthalpy of Dissociation)Varun GunduNo ratings yet
- Class 12 P - Block ElementsDocument33 pagesClass 12 P - Block ElementsIpsita SethiNo ratings yet
- bw33 Theory Sol eDocument11 pagesbw33 Theory Sol eivan manchewNo ratings yet
- Chemistry Under ConstructionDocument7 pagesChemistry Under Constructionsean goNo ratings yet
- TRENDS Part5Document2 pagesTRENDS Part5Sahar AnjumNo ratings yet
- The P Block ElementDocument11 pagesThe P Block Elementusingforadditionalpurpose100No ratings yet
- Magnetochemie SeminarzumPraktikum 2012Document25 pagesMagnetochemie SeminarzumPraktikum 2012SANKAR VNo ratings yet
- P Block Elements in PPT FormDocument138 pagesP Block Elements in PPT FormharshadNo ratings yet
- P-Block Elements (Class XII)Document63 pagesP-Block Elements (Class XII)gameofgreed876No ratings yet
- P Block 7 Nitrogen Family 1 PDFDocument21 pagesP Block 7 Nitrogen Family 1 PDFAdbhut DiwakarNo ratings yet
- P Block 7 Nitrogen Family 1 PDFDocument21 pagesP Block 7 Nitrogen Family 1 PDFbibha chaubeyNo ratings yet
- COORDINATION COMPOUNDS - Class Notes - JEE MindmapDocument22 pagesCOORDINATION COMPOUNDS - Class Notes - JEE Mindmapadsaditya24No ratings yet
- 6.P Block ElementsDocument24 pages6.P Block ElementsSSSSSSSSSSSSNo ratings yet
- Group 15 ElementsDocument24 pagesGroup 15 ElementsAkarshNo ratings yet
- GRP 15, 16 New P BlockDocument76 pagesGRP 15, 16 New P BlockVedantNo ratings yet
- Group 15 Elements: General IntroductionDocument21 pagesGroup 15 Elements: General IntroductionSirishaNo ratings yet
- CH 7Document36 pagesCH 7Tr Mazhar PunjabiNo ratings yet
- Coal Analysis + Price AdjusmentDocument46 pagesCoal Analysis + Price AdjusmentWahyoedyNo ratings yet
- ..-EAMCET-QR-Chemistry-Sr Chem-10.VA Group Elements - 171-175 - PDFDocument12 pages..-EAMCET-QR-Chemistry-Sr Chem-10.VA Group Elements - 171-175 - PDFPragyanshu ParadkarNo ratings yet
- KeepDocument5 pagesKeepAshwin KoradeNo ratings yet
- Chemistry Chapter 1.exercise 1ADocument28 pagesChemistry Chapter 1.exercise 1AAsifNo ratings yet
- VA GroupDocument3 pagesVA GroupvipulNo ratings yet
- UntitledDocument2,809 pagesUntitledOscar UrielNo ratings yet
- 10.VA Group Elements 171-175Document12 pages10.VA Group Elements 171-175eamcetmaterials100% (3)
- UAS Kimia Dasar - 1212914004 - Shidqi MFFDocument7 pagesUAS Kimia Dasar - 1212914004 - Shidqi MFFBig DaddyNo ratings yet
- Chapter-7: P-Block Element: Nitrogen Family (Group 15Document28 pagesChapter-7: P-Block Element: Nitrogen Family (Group 15Aditya VijayvargiyaNo ratings yet
- Part 2 Inorg. Struct.Document6 pagesPart 2 Inorg. Struct.Sahil RaundalNo ratings yet
- WEDNESDAY 12:00 - 2:00 PM: Oceña, Margarito Jr. ODocument8 pagesWEDNESDAY 12:00 - 2:00 PM: Oceña, Margarito Jr. ONivla GenesisNo ratings yet
- The P - Block Elements 2Document16 pagesThe P - Block Elements 2prateekNo ratings yet
- S Che CP CASS NEET-UG (Sol) ENG 2PDocument4 pagesS Che CP CASS NEET-UG (Sol) ENG 2PRaktim FactoryNo ratings yet
- Solution 2Document12 pagesSolution 2Varad DNo ratings yet
- Solutions - Iit-Jee-2011: Code: 2: Chemistry Paper - 1Document5 pagesSolutions - Iit-Jee-2011: Code: 2: Chemistry Paper - 1Chinedu H. DuruNo ratings yet
- P BLOCK Class 12Document26 pagesP BLOCK Class 12Parth BajajNo ratings yet
- Cajepe, Cherry May F. Bses 1a ChemistryDocument4 pagesCajepe, Cherry May F. Bses 1a ChemistryNilda FranciscoNo ratings yet
- Inorganic - Orders & Exceptions - 230121 - 191219Document40 pagesInorganic - Orders & Exceptions - 230121 - 191219scrbdddNo ratings yet
- Semester Test 2 MemoDocument9 pagesSemester Test 2 MemoMac'Ann Ditshego MashaoNo ratings yet
- ICSE Selina Solution For Class 9 Chemistry Chapter 1Document19 pagesICSE Selina Solution For Class 9 Chemistry Chapter 1ABHISHEK THAKURNo ratings yet
- Steps For Balancing Redox Reactions With The Reaction MethodDocument4 pagesSteps For Balancing Redox Reactions With The Reaction Methodkg4freeNo ratings yet
- P-Block 15 To 16 GroupDocument38 pagesP-Block 15 To 16 GroupBharti GoelNo ratings yet
- (2099) Lecture Notes P Block 15 16 E.pdf - TMPDocument39 pages(2099) Lecture Notes P Block 15 16 E.pdf - TMPRamJiPandeyNo ratings yet
- ANO2A Geometries Isomers 2018 PDFDocument40 pagesANO2A Geometries Isomers 2018 PDFJelte de WitNo ratings yet
- Answers of Exercise 1 (A)Document6 pagesAnswers of Exercise 1 (A)Lisa SinhaNo ratings yet
- HeterocyclicsDocument28 pagesHeterocyclicspawanaana100% (1)
- Chapter 3 f4 2019Document36 pagesChapter 3 f4 2019Leena bsb.No ratings yet
- New PDocument108 pagesNew Pjayesh soniNo ratings yet
- 9 - P-Block Elements PDFDocument27 pages9 - P-Block Elements PDFthinkiit86% (7)
- THE P - Block Elements-Anil-Hsslive PDFDocument19 pagesTHE P - Block Elements-Anil-Hsslive PDFKochuzNo ratings yet
- Phchem 1B - Quiz #1 - Chemical Nomenclature (Summer 2022)Document2 pagesPhchem 1B - Quiz #1 - Chemical Nomenclature (Summer 2022)Shopifyy ClothingNo ratings yet
- Name: Jave Jose M. Dela Cruz Section: BSCE 1-1 GradeDocument6 pagesName: Jave Jose M. Dela Cruz Section: BSCE 1-1 GradeWild RiftNo ratings yet
- Chapter 9 Periodicity Revision (Document5 pagesChapter 9 Periodicity Revision (Ting NicholasNo ratings yet
- Sprint+With+Kick P Block+ (Group+15 18) +in+One+Kick+ (29.9.2021)Document122 pagesSprint+With+Kick P Block+ (Group+15 18) +in+One+Kick+ (29.9.2021)Arman ArmanNo ratings yet
- Spektroskopi Ultraviolet & VisibelDocument39 pagesSpektroskopi Ultraviolet & VisibeladelasyafiraNo ratings yet
- Chapter-07: The P-Block ElementsDocument10 pagesChapter-07: The P-Block ElementsCheryl ChaudhariNo ratings yet
- Chapter 20 Worksheet Redox WSDocument4 pagesChapter 20 Worksheet Redox WSMostafa Ahmed100% (1)
- Cations/anions CL CO NO S PO CN Na NH MG Al PBDocument3 pagesCations/anions CL CO NO S PO CN Na NH MG Al PBJohnmarco RomeroNo ratings yet
- Critical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsFrom EverandCritical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsNo ratings yet