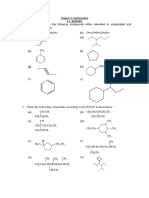
Professional Documents
Culture Documents
VPTS-3B 18-03-2021
Uploaded by
Aayush NagpalOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
VPTS-3B 18-03-2021
Uploaded by
Aayush NagpalCopyright:
Available Formats
Important Instructions:
1. Immediately fill in the particulars on this page of the Test Booklet with Blue or Black Ball Point Pen.
To mark answers on the OMR Sheet, use Blue or Black ball point pen only.
2. The Test Booklet consists of 180 questions. There are three sections in the question paper, Subject I, II and
III consisting of Physics (45 Questions), Chemistry (45 Questions), Botany (45 questions) & Zoology (45
questions) in each subject of equal weightage.
3. Each question is allotted 4 (four) marks for correct response. For each wrong answer, 1 (One) mark will be
deducted. No deduction from the total score will be made if no response is indicated for an item in the
answer sheet.
4. There is only one correct response for each question. Filling up more than one response in any question
will be treated as wrong response and marks for wrong response will be deducted accordingly as per
instruction 3 above.
5. No candidate is allowed to carry any text material, printed or written, mobile phone or any electronic
device, etc. inside the examination room/hall.
6. Do not fold or make any stray mark on the Answer Sheet.
7. The candidates are governed by all Rules and Regulations of the Examination body with regard to their
conduct in the Examination Hall. All cases of unfair means will be dealt with as per Rules and Regulations
of the Examination body.
SUBJECT SYLLABUS COVERED
PHYSICS Thermodynamics and Kinetic theory of gases, Waves and Oscillations
CHEMISTRY GOC- (I, II & III), Hydrocarbons, Environmental Chemistry
BOTANY Sexual reproduction in flowering plants, Principles of Inheritance and variation
ZOOLOGY Neural Control and Coordination, Chemical coordination and integration
Vidyamandir Classes
SECTION - II (CHEMISTRY) 180 MARKS
46. An alkene on treating with hot acidified 52. The addition of Br2 to trans-2-butene produces:
KMnO 4 gives succinic acid. The alkene is: (1) (+)-2, 3-Dibromobutane
I (1) 1-Butene (2) 2-Butene (2) (–)-2, 3-Dibromobutane
(3) 2-methylbutene (3) rac-2,3-Dibromobutane
(4) Cyclobutene (4) meso-2, 3-Dibromobutane
47. Maleic acid is allowed to react with Br2 in 53. 2-Butyne is treated with sodium in liquid NH 3 .
CCl 4 . The product formed is: The major product formed is:
(1) Dibromotartaric acid (1) cis-2-Butene (2) trans-2-Butene
(2) Dibromosuccinic acid (3) Butane (4) 1, 3-Butadiene
(3) ( ) Dibromosuccinic acid 54. In the following reaction
(4) meso-Dibromosuccinic acid C 2 H 2 2H O
X ƒ CH3CHO
HgSO /H SO
4 2 4
48. The addition of HBr to an alkene in the
What is X?
presence of peroxide is the example of:
(1) CH 3CH 2 OH
11 (1) Electrophilic addition reaction
(2) Nucleophilic addition reaction (2) CH 2 CHOH
(3) Free radical addition reaction (3) CH 3 CH 2 CHO
(4) The formation of carbocation as an (4) CH 3 O CH 3
intermediate Electrophilic substitution
reaction 55. In the reaction,
49. 2-Butene on reductive ozonolysis will give: (i) X
(1) Acetaldehyde CH3C C CH3
(ii) Zn/H O
2
(2) Acetic Acid H 3C C C CH3
(3) Mixture of acetaldehyde and acetic acid
(2 : 1 ratio)
I || ||
O O
X is:
(4) Mixture of acetaldehyde and acetic acid (1) HNO 3 (2) O2
(1 : 2 ratio) (3) O3 (4) KMnO 4
50. Which among the following alkenes will be 56. What is the product formed when acetylene
most stable? reacts with hypochlorous acid?
(1) Ethene (2) 2-Methylpropene (1) CH 3COCl
(3) 2, 3-Dimethyl-2-butene
(4) 2-Butene I (2) ClCH 2 CHO
(3) Cl CHCHO
51. The addition of HCl to 3, 3, 3-trichloropropene
(4) ClCH 2 COOH
give:
(1) Cl3CCH 2 CH 2 Cl 57. Which of the following is the most reactive
towards ring nitration?
I (2) Cl3CCH (Cl)CH 3
(1) Benzene (2) Mesitylene
(3) Cl2 CHCH (Cl)CH 2 Cl 1
(3) Toluene (4) m-Xylene
(4) Cl2 CHCH 2 CHCl 2
Page [7] SPACE FOR ROUGH WORK
Vidyamandir Classes
58. 1, 3-Butadiene when treated at low temperature CH CO H H
63.
3 3
with Br2 gives: H 2O
(1) 1, 4-dibromo-2-butene
I (2) 1, 3-dibromo-2-butene (1)
(3) 3, 4-dibromo-1-butene
(4) 2, 3-dibromo-2-butene I
59. The most reactive compound for electrophilic (2)
nitration is:
(3) Both of these
(1) Benzene
(4) None of these
(2) Nitrobenzene
H O
(3) Benzoic acid 64. A 2
H SO ,HgSO
2 4 4
(4) Toluene
60. What would be the product formed when 1-
Bromo-3-Chlorocyclubutane reacts with two
Identify A and B respectively:
equivalents of metallic sodium in water? I (1) Acetone, propanal
II (2) Acetone, acetone
(3) Propanal, propanal
(1) (2)
(4) Propanal, acetone
(3) (4)
(1)OsO
65. 4
61. The reagent(s) for the following compounds (2) NaHSO 4
is/are:
(1) alcoholic KOH
(2) Alcoholic KOH followed by NaNH 2 (1) (2)
(3) Aqueous KOH followed by NaNH2
(4) Zn /CH 2 OH
CH 2 (3) (4)
Br2
62. |
aq.NaOH
CH 2
Which of the following is not the expected 66. Which of the following reacts fastest with HCl?
product in the above reaction?
CH 2 Br CH 2Cl (1) (2)
(1) | (2) |
CH 2 Br CH 2Cl II
CH 2 Br CH 2 Br (3) (4)
(3) | (4) |
CH 2 Cl CH 2OH
Page [8] SPACE FOR ROUGH WORK
Vidyamandir Classes
67. The IUPAC name of: (1) 4 and 4 (2) 2 and 2
(3) 2 and 4 (4) 4 and 2
71. Maximum enolization take place of:
(1) CH 3COCH 3
(1) 3, 4-dimethyl pentanoyl chloride
10 (2) 1-chloro-1-oxo-2, 3-dimethyl pentane
(2) CH 3COCH 2 CHO
(3) 2-ethyl-3-methylbutanoylchloride 10 (3) CH 3COCH 2 COCH 3
(4) 2, 3-dimethyl pentanoyl chloride
68. (2R, 3R) – 2, 3-butanediol is: (4)
72. Tautomerism is not exhibited by:
(1)
(1)
(2)
10
(2)
(3)
(3)
(4)
(4)
69. Number of stereo isomers of tartaric acid
CH(OH)COOH
| 73. Number of optical isomers of X will be:
CH(OH)COOH
10 is: I
(1) 2 (2) 3
(3) 4 (4) 5 (1) 2 (2) 3
70. The compound, whose stereo-chemical formula (3) 4 (4) None of these
is written below, exhibits x geometrical isomers 74. Meso-tartaric acid is optically inactive due to:
and y optical isomers. ‘x’ and ‘y’ respectively (1) Two asymmetric carbon atom
are: (2) External compensation
10 10 (3) Molecular symmetry
(4) Molecular Asymmetry
Page [9] SPACE FOR ROUGH WORK
Vidyamandir Classes
75. Maleic acid and fumaric acid are: 80. Most stable alkene is:
(1) Position Isomers (1) CH 3CH CHCH 3
(2) Functional Isomers
10 (3) Geometrical isomers
(4) Metamers (2)
76. Molecular formula C 4 H 9 NH 2 shows how
many isomers of primary amine:
(3)
10 (1) 2 (2) 3
(3) 4 (4) 5
77. The chirality of the compound is: (4)
81. Relative stabilities of the following carbocation
will be in order:
(1) R (2) S
(3) Z (4) E
10
78. Electrophile N O 2 attacks the following
(1) I II III IV
(2) IV II III I
(3) I II III I
II (4) II IV III I
82. Dehydrobromination (—HBr) of the following
In which case N O 2 will be at meta position: in increasing order is:
(1) II, IV (2) I, II, III
(3) II, III (4) I only
79. Among the following compounds the
decreasing order of reactivity towards
I (1) I II III (2) III II I
(3) I =II III (4) III I = II
electrophilic substitution is:
83.
II Which is correct statement?
(1) II I III IV (1) A is formed by anti-addition and is Meso
(2) A is formed by syn-addition and is Meso
(2) III I II IV
(3) A is formed by anti-addition and is
(3) IV I II III
racemic
(4) I II III IV (4) A is formed by syn-addition and is
racemic
Page [10] SPACE FOR ROUGH WORK
Vidyamandir Classes
84. Arrange the following in increasing acid (3) Mixture of both (1) & (2)
strength: (4) None of these
87. In Victor Meyer’s method 0.2 gm of an organic
substance displaced 56 ml of air at STP the
molecular weight of the compound:
(1) 56 (2) 112
(3) 80 (4) 28
10 (1) II I III IV 88. The ammonia evolved from the treatment of
(2) II I IV III 0.30 g of an organic compound for the
estimation of nitrogen was passed in 100 mL of
(3) III IV II I
0.1 M sulphuric acid. The excess of acid
(4) IV III I II
required 20 mL of 0.5 M sodium hydroxide
85. Most stable free radical is: solution for complete neutralization. The
10
organic compound is:
(1) Urea
(1) (2) (2) Benzamide
(3) Acetamide
10
(4) Thiourea
89. How much sulphur is present in organic
(3) compound if on analysis 0.53 gm of this
(4) None of these compound gives 1.158 gm of BaSO 4 ?
10 (1) 10% (2) 15%
86. Product (s) of the following S N 1 reaction is/are:
(3) 20% (4) 30%
90. Which of these gases is not considered to be an
air pollutant?
(1) R-butan-2-ol (1) NO 2 (2) SO 2
(2) S-butan-2-ol (3) O3 (4) CO
Page [11] SPACE FOR ROUGH WORK
Vidyamandir Classes
End of (VPTS-3B) PRACTICE TEST SERIES-3B | NEET-2021
Page [20] SPACE FOR ROUGH WORK
You might also like
- CHEMISTRY O LEVEL (FORM THREE) - MOLE CONCEPT (PDF)Document14 pagesCHEMISTRY O LEVEL (FORM THREE) - MOLE CONCEPT (PDF)neveti.avorel50% (2)
- Chap 2 Material Balance Non-Reactive SystemDocument40 pagesChap 2 Material Balance Non-Reactive SystemAndreas LarssonNo ratings yet
- Neet Full Test-3Document21 pagesNeet Full Test-3vasteducationalNo ratings yet
- Cls Jeead-17-18 Xi Che Target-4 Set-2 Chapter-13Document34 pagesCls Jeead-17-18 Xi Che Target-4 Set-2 Chapter-13Jotiraj Parihar50% (2)
- Xicbse Che Haloalkanes Haloarenes 2 QPDocument3 pagesXicbse Che Haloalkanes Haloarenes 2 QPtanishkakannan3253No ratings yet
- Alkenes2 CNBFDocument19 pagesAlkenes2 CNBFAditya RaiNo ratings yet
- HydrocarbonDocument15 pagesHydrocarbonzohaibsalamNo ratings yet
- 10 Hydrocarbons: AssignmentDocument6 pages10 Hydrocarbons: AssignmentalarmbarbarNo ratings yet
- HydrocarbonDocument6 pagesHydrocarbonSwarnav ChatterjeeNo ratings yet
- XII NEET-2021-22: The Leader in Chemistry .....Document4 pagesXII NEET-2021-22: The Leader in Chemistry .....Rohan BholeNo ratings yet
- Jee-Main ChemistryDocument5 pagesJee-Main ChemistryAbhishek SaravananNo ratings yet
- Neet 1 Chemistry - PMDDocument7 pagesNeet 1 Chemistry - PMDsakif sNo ratings yet
- Hydrocarbons: SolutionsDocument34 pagesHydrocarbons: SolutionsAjay SainiNo ratings yet
- Chemistry (Question Paper)Document7 pagesChemistry (Question Paper)SarangShekokarNo ratings yet
- Christ King Academy: Subject:CHEMISTRY Test Max Marks: 30 General Organic ChemistryDocument5 pagesChrist King Academy: Subject:CHEMISTRY Test Max Marks: 30 General Organic ChemistryajaybolarNo ratings yet
- Alkanes - Alkenes - Alkynes - DPP 3Document3 pagesAlkanes - Alkenes - Alkynes - DPP 3Vishal_93100% (1)
- Chemistry Max Marks: 100: (Single Correct Answer Type)Document6 pagesChemistry Max Marks: 100: (Single Correct Answer Type)Gowri ShankarNo ratings yet
- 12th NEET ANSWERS - 30 - 04 - 2023Document9 pages12th NEET ANSWERS - 30 - 04 - 2023studyloverx1234No ratings yet
- Part (B) : Chemistry: Major Test - 4 (Main) Chemistry (CodeDocument12 pagesPart (B) : Chemistry: Major Test - 4 (Main) Chemistry (CodeAmit HhcbjNo ratings yet
- CU-2020 B.Sc. (Honours) Chemistry Semester-III Paper-CC-7 QPDocument4 pagesCU-2020 B.Sc. (Honours) Chemistry Semester-III Paper-CC-7 QPbuntyckbtNo ratings yet
- Carboxylic AcidDocument6 pagesCarboxylic Acidhareharanbt22No ratings yet
- HydrocarbonDocument15 pagesHydrocarbonChandrika AnchanNo ratings yet
- LT - Batch A - Unit Test - 6 - CHE & BOT - 23.03.2023 - A Type PDFDocument16 pagesLT - Batch A - Unit Test - 6 - CHE & BOT - 23.03.2023 - A Type PDFVENUGOPALARAONo ratings yet
- Halo DerivativesDocument5 pagesHalo DerivativesBHASWATI MANDALNo ratings yet
- Bitsat 2010 PaperDocument34 pagesBitsat 2010 PaperYumit MorwalNo ratings yet
- C Ch-22 HydrocarbonsDocument8 pagesC Ch-22 HydrocarbonsYOGENDRA singhNo ratings yet
- Inorganic DPP PDFDocument3 pagesInorganic DPP PDFashutosh99878No ratings yet
- PRACTICE SHEET - 02 (Chemistry) : Basic Concept of Organic (IUPAC, Isomerism)Document5 pagesPRACTICE SHEET - 02 (Chemistry) : Basic Concept of Organic (IUPAC, Isomerism)ABD 17No ratings yet
- Neet Full Test-2Document20 pagesNeet Full Test-2vasteducationalNo ratings yet
- BCHCT 133Document16 pagesBCHCT 133moviesmania.help4uNo ratings yet
- 134 PDFDocument12 pages134 PDFNaman VatsNo ratings yet
- Final Touch (RCC Do / Die Questions)Document24 pagesFinal Touch (RCC Do / Die Questions)harita shinde100% (1)
- A (2e, 4e) B (2Z, 4Z) C (2Z, 4e) D (2e, 4Z)Document1 pageA (2e, 4e) B (2Z, 4Z) C (2Z, 4e) D (2e, 4Z)Agatha chilesheNo ratings yet
- Eamcet Part Test - 5Document6 pagesEamcet Part Test - 5udaysrinivasNo ratings yet
- Mathongo Jee Main 2015Document28 pagesMathongo Jee Main 2015rishithhr rajeevNo ratings yet
- Organic Chemistry (Alkyl Had., Stereo., Aromat.) (160 Items)Document17 pagesOrganic Chemistry (Alkyl Had., Stereo., Aromat.) (160 Items)S AdiaNo ratings yet
- 6.DAY-8 CHE - Organic Chemistry Electron Migration Effects & Reagents - 25-05-2020 PDFDocument7 pages6.DAY-8 CHE - Organic Chemistry Electron Migration Effects & Reagents - 25-05-2020 PDFRamakrishna ReddyNo ratings yet
- Carbonyl Compounds Day-4 W.SDocument12 pagesCarbonyl Compounds Day-4 W.SLalitha MarimuthuNo ratings yet
- CHEMISTRYCET-16thOCT Ixm5pzgcfy8k2ejcDocument7 pagesCHEMISTRYCET-16thOCT Ixm5pzgcfy8k2ejcanuNo ratings yet
- CL H CL H CL H CL H P) Q) : X H Monohalogenated ProductDocument12 pagesCL H CL H CL H CL H P) Q) : X H Monohalogenated ProductDivya KhandelwalNo ratings yet
- Test - 10 (B) - Nomenclature & Isomerism + Reaction Mechanism I & II and Hydrocarbon (Paper)Document5 pagesTest - 10 (B) - Nomenclature & Isomerism + Reaction Mechanism I & II and Hydrocarbon (Paper)rahulkaushikNo ratings yet
- XI-Chemistry Chapter Test-13-HydrocarbonsDocument4 pagesXI-Chemistry Chapter Test-13-Hydrocarbonscakof67215No ratings yet
- Universiti Kuala Lumpur: Malaysian Institute of Chemical & Bioengineering TechnologyDocument4 pagesUniversiti Kuala Lumpur: Malaysian Institute of Chemical & Bioengineering TechnologyNufar MohmdNo ratings yet
- Hydrocarbons Work SheetDocument30 pagesHydrocarbons Work SheettarunvishalgrNo ratings yet
- 62c2d57c17f4c30011d5e108 - ## - Alcohol, Phenol & Ether - DPP 01Document3 pages62c2d57c17f4c30011d5e108 - ## - Alcohol, Phenol & Ether - DPP 01Prarabdh TiwariNo ratings yet
- Reaction Mechanism IIDocument21 pagesReaction Mechanism IIFilmode100% (2)
- Reaction Mechanism-IiDocument21 pagesReaction Mechanism-IiKaustav BanikNo ratings yet
- Chapter 5.1Document3 pagesChapter 5.1Aliff AmirudinNo ratings yet
- Halo AlkaneDocument4 pagesHalo Alkanetechnicalgamerz818No ratings yet
- 01 HydrocarbonsDocument6 pages01 HydrocarbonslingarajugowdaNo ratings yet
- Tutorial Kit (Chemistry-200 L) - Vol. 2 PDFDocument84 pagesTutorial Kit (Chemistry-200 L) - Vol. 2 PDFLucienne IrianaNo ratings yet
- Alkanes Alkenes AlkynesDocument10 pagesAlkanes Alkenes AlkynesPanda Boy100% (2)
- Aliphatic HydrocarbonsDocument11 pagesAliphatic HydrocarbonsRishabhNo ratings yet
- Hydrocarbons Jumbo Sheet by MKA SirDocument44 pagesHydrocarbons Jumbo Sheet by MKA SirRahul SinghNo ratings yet
- Organic Chemistry For Medicine Chapter 3Document39 pagesOrganic Chemistry For Medicine Chapter 3أمال داودNo ratings yet
- CB and APEDocument4 pagesCB and APEAnubrata SarkarNo ratings yet
- HydrocarbonDocument29 pagesHydrocarbondhawang40No ratings yet
- Iitian'S Hub: Assignment # 1 General Organic Chemistry ChemistryDocument11 pagesIitian'S Hub: Assignment # 1 General Organic Chemistry ChemistrySAHILI RANENo ratings yet
- Questions in TEST BOOKLET: 100 MAX MARKS: 400 (+4/-1) : Minor 9Document8 pagesQuestions in TEST BOOKLET: 100 MAX MARKS: 400 (+4/-1) : Minor 9Sanskar SahuNo ratings yet
- Organics, Synthesis and Enthalpy MSDocument47 pagesOrganics, Synthesis and Enthalpy MSAnh Nguyễn VănNo ratings yet
- Alka NetDocument13 pagesAlka Netjonida88No ratings yet
- F.K. Hwang: A T & T Beu Laboratories, Murray Hill, NJ 07974, U.S.ADocument9 pagesF.K. Hwang: A T & T Beu Laboratories, Murray Hill, NJ 07974, U.S.AAayush NagpalNo ratings yet
- 1 s2.0 S0370269308004589 MainDocument5 pages1 s2.0 S0370269308004589 MainSurender KumarNo ratings yet
- Coherent Electrical Control of A Single High-Spin Nucleus in SiliconDocument11 pagesCoherent Electrical Control of A Single High-Spin Nucleus in SiliconAayush NagpalNo ratings yet
- Physics Letters B: Shreyashi Chakdar, Kirtiman Ghosh, S. NandiDocument6 pagesPhysics Letters B: Shreyashi Chakdar, Kirtiman Ghosh, S. NandiAayush NagpalNo ratings yet
- 10 3847@1538-4357@ab5d35 PDFDocument12 pages10 3847@1538-4357@ab5d35 PDFAayush NagpalNo ratings yet
- Loss-Free Excitonic Quantum Battery: Junjie Liu, Dvira Segal, and Gabriel HannaDocument12 pagesLoss-Free Excitonic Quantum Battery: Junjie Liu, Dvira Segal, and Gabriel HannaAayush NagpalNo ratings yet
- Physioex Lab Report: Pre-Lab Quiz ResultsDocument3 pagesPhysioex Lab Report: Pre-Lab Quiz ResultsNicole de LeonNo ratings yet
- Aeroquip HoseDocument0 pagesAeroquip Hosegbm2246No ratings yet
- Hempadur Primer 1530yDocument4 pagesHempadur Primer 1530yRodrigo CondorettyNo ratings yet
- DLL Q1 Lesson 5 Concentration of SolutionsDocument3 pagesDLL Q1 Lesson 5 Concentration of SolutionsMa. Elizabeth Cusi100% (1)
- 28 MBD City Blo ListDocument30 pages28 MBD City Blo ListTusharr AhujaNo ratings yet
- Semi F21-95 Classification of Airborne Molecular ContaminantDocument4 pagesSemi F21-95 Classification of Airborne Molecular ContaminantzyatikNo ratings yet
- Oracal 970 RA Premium Special EffectDocument2 pagesOracal 970 RA Premium Special EffectIonut ValentinNo ratings yet
- ON General Chemistry: By: Nativity Ivy A. Mugas, RPHDocument22 pagesON General Chemistry: By: Nativity Ivy A. Mugas, RPHRoberto Velasco MabulacNo ratings yet
- Paintin - TITAS-383-PI-PI-PRO-xxxxxxxx Rev. 00Document34 pagesPaintin - TITAS-383-PI-PI-PRO-xxxxxxxx Rev. 00ismail batinNo ratings yet
- Basf Masterpolyheed 3532ae TdsDocument2 pagesBasf Masterpolyheed 3532ae TdsVisJadNo ratings yet
- Adrienne Kress - The Lost Ones (Retail)Document251 pagesAdrienne Kress - The Lost Ones (Retail)Hayden Refigio100% (2)
- Exercises: H, HT, HHT, HHHDocument8 pagesExercises: H, HT, HHT, HHHElbtbraNo ratings yet
- Properties of Baked Foams From Citric Acid Modified Cassava Starchand Native Cassava Starch BlendsDocument6 pagesProperties of Baked Foams From Citric Acid Modified Cassava Starchand Native Cassava Starch BlendsShamir Jose ContrerasNo ratings yet
- Ce 112 Module 1 Intro and Matter - EnergyDocument29 pagesCe 112 Module 1 Intro and Matter - EnergyjeremytalenssNo ratings yet
- Nematode Sterilazion Method Mercuric StreptomycinDocument6 pagesNematode Sterilazion Method Mercuric StreptomycinFernanda gNo ratings yet
- MSDS R6Document3 pagesMSDS R6jafarptrNo ratings yet
- Moudling Operation GSIC Process: MouldingDocument13 pagesMoudling Operation GSIC Process: MouldingBalakumaran MurugesanNo ratings yet
- Summer Training Repot by Shubham MishraDocument114 pagesSummer Training Repot by Shubham MishraAnkit Singh100% (1)
- Huang Et Al. 2017Document8 pagesHuang Et Al. 2017Yoni AtmaNo ratings yet
- Sec02 1Document19 pagesSec02 1Mahmoud NaDaaNo ratings yet
- Sikagrout®-114 Ae: Product Data SheetDocument3 pagesSikagrout®-114 Ae: Product Data SheetAlexander Jonas Zach ValdrizNo ratings yet
- Ion Exchange, Molecular Sieve, AffinityDocument11 pagesIon Exchange, Molecular Sieve, AffinityNofrizalNo ratings yet
- APAR Conductors BrochureDocument178 pagesAPAR Conductors BrochureAshish bhattNo ratings yet
- PHD Thesis On Organisational LearningDocument4 pagesPHD Thesis On Organisational Learningbskb598g100% (1)
- MSDS TCCA Brataco 2019Document3 pagesMSDS TCCA Brataco 2019juwitapermata33No ratings yet
- Plan Design Lab SampleDocument3 pagesPlan Design Lab SampleDumissa MelvilleNo ratings yet
- Olive Oil and Clove Oil Based Nanoemulsion For Topical Delivery of Terbinafine Hydrochloride in Vitro and Ex Vivo EvaluationDocument14 pagesOlive Oil and Clove Oil Based Nanoemulsion For Topical Delivery of Terbinafine Hydrochloride in Vitro and Ex Vivo EvaluationRaghavendra NaveenNo ratings yet
- Chapter 1 - The Mole CalculationsDocument40 pagesChapter 1 - The Mole Calculationsredifentsemosiane6No ratings yet