Professional Documents
Culture Documents
Tutorial 1
Uploaded by
SUREINTHARAAN A/L NATHAN / UPMCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Tutorial 1
Uploaded by
SUREINTHARAAN A/L NATHAN / UPMCopyright:
Available Formats
ECH3126: Tutorial 1
Question 1
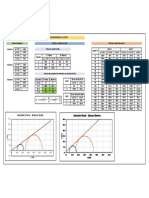
The vapour-pressure data are given below for the system hexane-octane:
Vapor pressure
n-Hexane n-Octane
T (F) T (C) kPa mm Hg kPa mm Hg
155.7 68.7 101.3 760 16.1 121
175 79.4 136.7 1025 23.1 173
200 93.3 197.3 1480 37.1 278
225 107.2 284.0 2130 57.9 434
258.2 125.7 456.0 3420 101.3 760
(a) Using Raoult’s law, calculate and plot the x-y data at a total pressure of 101.32 kPa.
(b) Plot the boiling point diagram
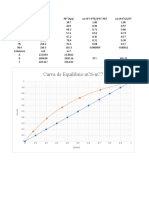
Question 2
A mixture of 100 mol containing 60 mol % m-pentane and 40 mol % n-heptane is vaporized at
101.32 kPa abs pressure until 40 mol of vapour and 60 mol of liquid in equilibrium with each
other are produced. This occurs in a single-stage system, and the vapour and liquid are kept in
contact with each other until vaporization is complete. The equilibrium data are given below.
Calculate the composition of the vapour and the liquid.
x y
1.0 1.0
0.867 0.984
0.594 0.925
0.398 0.836
0.254 0.701
0.145 0.521
0.059 0.271
0 0
You might also like
- Sample For Solution Manual Introduction To Chemical Engineering Thermodynamics 9th International Edition by Smith and Van Ness and AbbottDocument34 pagesSample For Solution Manual Introduction To Chemical Engineering Thermodynamics 9th International Edition by Smith and Van Ness and AbbottEnoch AffulNo ratings yet
- Mass Transfer PartDocument36 pagesMass Transfer Partoctoviancletus79% (39)
- Tutorial 2Document3 pagesTutorial 2Syakirin SpearsNo ratings yet
- Tutorial 2 PDFDocument3 pagesTutorial 2 PDFWan ShamNo ratings yet
- CHE 411 Exam 1-2008Document1 pageCHE 411 Exam 1-2008aleeyakamalNo ratings yet
- CHE 42 - Problem Set 2 - Flash DistillationDocument4 pagesCHE 42 - Problem Set 2 - Flash DistillationROMELIE GABALLO ALBIANo ratings yet
- Component-A (MVC)Document16 pagesComponent-A (MVC)westewrNo ratings yet
- GRP 4 AssDocument6 pagesGRP 4 AssPRINCESS DIANNE DUG-ANo ratings yet
- PrinGeotec - Tarea Módulo 9 - Solu - 1096Document1 pagePrinGeotec - Tarea Módulo 9 - Solu - 1096John Javier Pajaro LastraNo ratings yet
- Chem Eng Practice ProblemsDocument2 pagesChem Eng Practice ProblemsfreescribdNo ratings yet
- Block 4 - Tutorial 4 SolutionsDocument6 pagesBlock 4 - Tutorial 4 SolutionsKarabo Kaytee DhlaminiNo ratings yet
- Assignment Question paper-III Year-2021Document4 pagesAssignment Question paper-III Year-2021Mothish SivakumarNo ratings yet
- Conversion Factors and Values of The Gas Constant: Appendix ADocument27 pagesConversion Factors and Values of The Gas Constant: Appendix Aمرتضى كاظم غانمNo ratings yet
- I Componente A B C Psat (Kpa)Document3 pagesI Componente A B C Psat (Kpa)Katerine Ostos ArellanoNo ratings yet
- CSTR Unit Ops LabDocument7 pagesCSTR Unit Ops LabKelly Sheine SisonNo ratings yet
- PSRK: A Group Contribution Equation of State Based On UNIFACDocument15 pagesPSRK: A Group Contribution Equation of State Based On UNIFACShunsuke NiwakkoNo ratings yet
- Fundamentals of Chemical Engineering Thermodynamics 1St Edition Dahm Solutions Manual Full Chapter PDFDocument57 pagesFundamentals of Chemical Engineering Thermodynamics 1St Edition Dahm Solutions Manual Full Chapter PDFadelehuu9y4100% (13)
- Group 1 - ALEJANO - DEZOLLER - GRATIS - BATCH REACTORDocument8 pagesGroup 1 - ALEJANO - DEZOLLER - GRATIS - BATCH REACTORJohn Frix AlejanoNo ratings yet
- Solved Problems PDFDocument6 pagesSolved Problems PDFMayank PrasadNo ratings yet
- Sistema: Metanol (1) / Acetonidrilo (2) Modelo UNIFAC: Ganma 1Document5 pagesSistema: Metanol (1) / Acetonidrilo (2) Modelo UNIFAC: Ganma 1Carlos Coronado LezmaNo ratings yet
- Batch Distillation ReportDocument14 pagesBatch Distillation ReportMahmoud HendawyNo ratings yet
- Jhardy MidtermDocument10 pagesJhardy MidtermJacob HardyNo ratings yet
- PBRDocument19 pagesPBRdarvyneeNo ratings yet
- HMB 1Document3 pagesHMB 1Besan LaduNo ratings yet
- Diagrama de Eq N-Hexano - N HeptanoDocument5 pagesDiagrama de Eq N-Hexano - N HeptanoLaura ReyesNo ratings yet
- Branzuela, Candar, Lasola - Nonideal VLE CalculationsDocument38 pagesBranzuela, Candar, Lasola - Nonideal VLE CalculationsJames DarelNo ratings yet
- Tutorial 1 - Review of ThermodynamicsDocument2 pagesTutorial 1 - Review of ThermodynamicsAdruNo ratings yet
- Bag. KelvinDocument9 pagesBag. KelvinkhairinashNo ratings yet
- Woodman 1925Document4 pagesWoodman 1925edmentzNo ratings yet
- Chapter 3 - Laboratory Analysis of Reservoir - 2019 - Reservoir Engineering Han PDFDocument44 pagesChapter 3 - Laboratory Analysis of Reservoir - 2019 - Reservoir Engineering Han PDFCarolineNo ratings yet
- DC and AC CircuitsDocument18 pagesDC and AC Circuitswei.cheng.auNo ratings yet
- Master Complex Columns and Four Assumptions Problems 2020 Set - 5Document7 pagesMaster Complex Columns and Four Assumptions Problems 2020 Set - 5vikyappleNo ratings yet
- Coefficient of Thermal ExpansionDocument6 pagesCoefficient of Thermal ExpansionemiljuchiacNo ratings yet
- Example 9.4 Binary FlashDocument4 pagesExample 9.4 Binary FlashWaleed WaleedNo ratings yet
- CHE 411 Assignment 4-2008 PDFDocument1 pageCHE 411 Assignment 4-2008 PDFsuzie annNo ratings yet
- CHE 312 - Spring 2024 - HPR - 1Document3 pagesCHE 312 - Spring 2024 - HPR - 1Yaren ErelNo ratings yet
- Composition of Methanol-Water Batch Distillation: Prepared By: Jason Hixson Don Scott Michael Hickey September 20, 2005Document28 pagesComposition of Methanol-Water Batch Distillation: Prepared By: Jason Hixson Don Scott Michael Hickey September 20, 2005bakhtyar21No ratings yet
- Executive Summary: Design ReportDocument3 pagesExecutive Summary: Design ReportAhmed YounisNo ratings yet
- PSRK Group Contribution Equation of State: Revision and Extension IIIDocument14 pagesPSRK Group Contribution Equation of State: Revision and Extension IIIAndrés F. CáceresNo ratings yet
- Calibration Curve: 1. A Hygrometer, Which Measures The Amount of Moisture in A Gas Stream, Is To Be CalibratedDocument35 pagesCalibration Curve: 1. A Hygrometer, Which Measures The Amount of Moisture in A Gas Stream, Is To Be Calibratedmichsantos0% (1)
- Conc. vs. Enthalpy Plot.: 2.5 X y X y y XDocument8 pagesConc. vs. Enthalpy Plot.: 2.5 X y X y y XakhilNo ratings yet
- Lab AssignmentsDocument7 pagesLab AssignmentsMohamed Omar IbrahimNo ratings yet
- Specific Heat Capacities of AirDocument13 pagesSpecific Heat Capacities of AirNguyen ChuyenNo ratings yet
- DIAGRAMA nC6-nC7Document2 pagesDIAGRAMA nC6-nC7Aylin Portillo OliveraNo ratings yet
- Intro To PVTDocument19 pagesIntro To PVTFernando OlaveoNo ratings yet
- The Model: A UNIFAC-Based Equation of State Model For Prediction of Gas Solubility and Vapor-Liquid Equilibria at Low and High PressuresDocument10 pagesThe Model: A UNIFAC-Based Equation of State Model For Prediction of Gas Solubility and Vapor-Liquid Equilibria at Low and High PressuresFlor de LeonNo ratings yet
- Session: Modeling, Simulation and OptimizationDocument31 pagesSession: Modeling, Simulation and Optimizationcygnumvignesh89No ratings yet
- DATOS PARA DIFERENTES MEZCLAS 1butanol Agua Acetona 2 PropanolDocument9 pagesDATOS PARA DIFERENTES MEZCLAS 1butanol Agua Acetona 2 PropanolAndresDiazNo ratings yet
- Mass Transfer Lab 1Document9 pagesMass Transfer Lab 1Fahad kamranNo ratings yet
- Balance de Materia 1Document12 pagesBalance de Materia 1diego veyzagaNo ratings yet
- Batch Reactor PDFDocument29 pagesBatch Reactor PDFSaranya KannanNo ratings yet
- Solid-Liquid Equilibrium in Binay System: Department of ChemistryDocument11 pagesSolid-Liquid Equilibrium in Binay System: Department of ChemistryLuluaNo ratings yet
- Solubility of Carbon Monoxide in N-Hexane Between and K and CO Pressures Up BarDocument4 pagesSolubility of Carbon Monoxide in N-Hexane Between and K and CO Pressures Up BarTiên PhạmNo ratings yet
- HydrocrabonDocument12 pagesHydrocrabonsalman hussainNo ratings yet
- CHE 42 - Problem Set 4 - Column DistillationDocument2 pagesCHE 42 - Problem Set 4 - Column DistillationROMELIE GABALLO ALBIANo ratings yet
- Vle G8Document12 pagesVle G8jjain26904No ratings yet
- HW 5 A 2017Document3 pagesHW 5 A 2017maxmNo ratings yet
- Physics Ia FinalDocument11 pagesPhysics Ia FinalscarletNo ratings yet
- In Calibrating A 10Document4 pagesIn Calibrating A 10GrenlyKerehNo ratings yet
- Tutorial 1Document1 pageTutorial 1SUREINTHARAAN A/L NATHAN / UPMNo ratings yet
- Topic 4 Linear Algebraic Equations and MatricesDocument25 pagesTopic 4 Linear Algebraic Equations and MatricesSUREINTHARAAN A/L NATHAN / UPMNo ratings yet
- ECH 3708 Week 2 - Properties of Pure SubstanceDocument86 pagesECH 3708 Week 2 - Properties of Pure SubstanceSUREINTHARAAN A/L NATHAN / UPMNo ratings yet
- Topic 9Document18 pagesTopic 9SUREINTHARAAN A/L NATHAN / UPMNo ratings yet
- Topic 10Document15 pagesTopic 10SUREINTHARAAN A/L NATHAN / UPMNo ratings yet
- Topic 8Document16 pagesTopic 8SUREINTHARAAN A/L NATHAN / UPMNo ratings yet