Professional Documents
Culture Documents
Explain Activity 1.10 Class 10 NCERT Science
Uploaded by
sciencee20090 ratings0% found this document useful (0 votes)
5 views2 pagesOriginal Title
1.10
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
5 views2 pagesExplain Activity 1.10 Class 10 NCERT Science
Uploaded by
sciencee2009Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
Explain Activity 1.
10 Class 10 NCERT Science
Caution: This activity needs the teacher’s assistance.
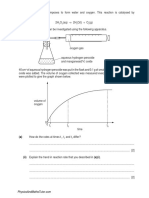
Activity- Take about 3 ml of sodium sulphate solution in a test tube.
In another test tube. take about 3 ml of barium chloride solution.
Mix the two solutions
What do you observe?
Explain Activity 1.10 Class 10 NCERT Science
Observation- in this reaction. it is observed that an insoluble white solid substance is formed.
Explanation
When sodium sulphate and barium chloride react, a double displacement reaction takes place.
After the exchange of ions between compounds, a white solid substance of barium sulphate is formed and
sodium chloride remains in the solution.
Conclusion- The insoluble white substance formed is known as a precipitate.
Na2SO4(aq) + BaCI2(aq) → BaSO4(s) + 2NaCI(aq)
This is also known as Precipitation Reaction.
Questions based on the activity 1.10
1. What is a precipitate?
Ans – Precipitate is an insoluble solid substance.
2. What is a precipitation reaction?
Ans – The reaction in which an insoluble solid substance is formed is called a precipitation reaction.
3. What is the chemical name of the precipitate formed during the reaction?
Ans- Barium sulphate
4. Write the chemical formula of the precipitate formed during the reaction?
And – BaSO4
5. Write the chemical reaction that takes place in activity 1.10?
Ans - Na2SO4(aq) + BaCI2(aq) → BaSO4(s) + 2NaCI(aq)
MCQ
1. Name the reaction involved when sodium sulphate is added to barium chloride?
(A) Decomposition reaction (B) Double displacement reaction
(C) Combination reaction (D) Single displacement reaction
2.What are the products of the reaction between sodium sulphate and barium chloride?
(A) Sodium chloride and barium sulphate (B) Barium sulphate
(C) Sodium sulphide and barium chloride (D) Barium chloride only
3.What is the colour of the precipitate formed during this reaction?
(A) White (B) Blue (C) Green (D) Yellow
4.What is the precipitate formed when sodium sulphate is added to barium chloride?
(A) Barium sulphate (B) Sodium chloride (C) Both A and B (D) None of the above
5. Which type of reaction takes place in the activity 1.10?
(A) Precipitation reaction (B) Neutralisation reaction (C) Oxidation reaction (D) Acid – base
reaction
You might also like
- Chemistry Cheat SheetDocument10 pagesChemistry Cheat Sheetbrook93% (40)
- Explain Activity 1.3 Class 10 NCERT ScienceDocument1 pageExplain Activity 1.3 Class 10 NCERT Sciencesciencee2009No ratings yet
- Chemical Reactions and Equations - Daily Home Assignment 05 (Of Lec 06)Document2 pagesChemical Reactions and Equations - Daily Home Assignment 05 (Of Lec 06)Samyak SethiNo ratings yet
- TP03 ChemicalDocument6 pagesTP03 Chemicalkamaldwivedi99No ratings yet
- NCERT Exemplar Solution Class 10 Science Chapter 1Document18 pagesNCERT Exemplar Solution Class 10 Science Chapter 1JenifarNishaNo ratings yet
- MSB Examplar Questions Class XDocument21 pagesMSB Examplar Questions Class XJeevansh WadhwaNo ratings yet
- School Data Adis1 Assignment 20660 GR 10 Revision Ans KeyDocument9 pagesSchool Data Adis1 Assignment 20660 GR 10 Revision Ans Keysharon VijuNo ratings yet
- Chemical Reactions and Equations - DHA 05 Discussion NotesDocument8 pagesChemical Reactions and Equations - DHA 05 Discussion NotesSamyak SethiNo ratings yet
- Ncert Solutions Class 10 Science Chapter 1Document11 pagesNcert Solutions Class 10 Science Chapter 1pk rNo ratings yet
- Aakash Institute: NCERT Solution For Class 10 Science Chapter 1 Chemical Reactions and EquationsDocument8 pagesAakash Institute: NCERT Solution For Class 10 Science Chapter 1 Chemical Reactions and EquationsSuneethaNo ratings yet
- 10th Science Byjus SolutionsDocument159 pages10th Science Byjus SolutionsChinmay B PNo ratings yet
- NCERT Solution For Class 10 Science Chapter 1 Chemical Reactions and EquationsDocument8 pagesNCERT Solution For Class 10 Science Chapter 1 Chemical Reactions and Equationssamiksha choudharyNo ratings yet
- Chem RXN and EqnDocument5 pagesChem RXN and EqnVaishnavi RajgopalNo ratings yet
- Chemical Equations 1Document7 pagesChemical Equations 1जggerNaut ClassesNo ratings yet
- 10 Science Ncert ch1 PDFDocument11 pages10 Science Ncert ch1 PDFArush YadavNo ratings yet
- Chemical Reactions and Equations - Daily Home Assignment 05 (Of Lecture 06) - (Udaan 2024)Document3 pagesChemical Reactions and Equations - Daily Home Assignment 05 (Of Lecture 06) - (Udaan 2024)shubhsomvanshi2087No ratings yet
- CLASS X CHEMISTRY question-988058-MCQ-PART2Document11 pagesCLASS X CHEMISTRY question-988058-MCQ-PART2abiniveshofficial4708No ratings yet
- Tinywow Science Class 10 Ch-1 Assignmnet 51714477Document3 pagesTinywow Science Class 10 Ch-1 Assignmnet 51714477HarishNo ratings yet
- Chemistry Chemical Equation and ReactionDocument7 pagesChemistry Chemical Equation and Reactionsmriti khannaNo ratings yet
- Acid Bases and Salts Lakhmir SinghDocument69 pagesAcid Bases and Salts Lakhmir SinghAishwaria MurthyNo ratings yet
- Chemistry 11 Practice Questions For The Solutions 4 Powerpoint Solution StoichiometryDocument2 pagesChemistry 11 Practice Questions For The Solutions 4 Powerpoint Solution Stoichiometryesn_kNo ratings yet
- ACIDSDocument6 pagesACIDSChahek KalraNo ratings yet
- Important Questions Class 10 Science Chapter 1Document21 pagesImportant Questions Class 10 Science Chapter 1Darshuram DudheNo ratings yet
- MAQ Class 10Document25 pagesMAQ Class 10Evil GamerNo ratings yet
- Selina Sol Concise Chem Class 10 CH 11Document7 pagesSelina Sol Concise Chem Class 10 CH 11StNo ratings yet
- CHAP 1.pmd5Document4 pagesCHAP 1.pmd5Ezhil CNo ratings yet
- Science PYQDocument82 pagesScience PYQgovidaswamygNo ratings yet
- 10 CT-05Document1 page10 CT-05Uday Prakash SahuNo ratings yet
- 1.chemical Reactions & Equations - CBSE PYQDocument8 pages1.chemical Reactions & Equations - CBSE PYQdeepthividhya0129No ratings yet
- Science Class 10 Complete BooksDocument76 pagesScience Class 10 Complete BooksTemsuyanger JamirNo ratings yet
- TIE, NIE, Selective Percipitation and Solution Stoichiometry PracticeDocument2 pagesTIE, NIE, Selective Percipitation and Solution Stoichiometry PracticeSerena ChinNo ratings yet
- SCH4C Types of Chemical ReactionsDocument8 pagesSCH4C Types of Chemical ReactionsSteve M HallNo ratings yet
- Ch1 MCQ Sheet PadhleDocument7 pagesCh1 MCQ Sheet PadhleAmit KumarNo ratings yet
- Important Question ICSE 2010 Class 10th Acids Bases Salts BDocument8 pagesImportant Question ICSE 2010 Class 10th Acids Bases Salts BYash KapoorNo ratings yet
- NCERT Solutions For Class 10 March 29 Science Chapter 1 Chemical Reactions and EquationsDocument9 pagesNCERT Solutions For Class 10 March 29 Science Chapter 1 Chemical Reactions and EquationsMohd Abuzar HasanNo ratings yet
- Ncert Solution Chapter - 1Document8 pagesNcert Solution Chapter - 1joydeep17590No ratings yet
- 2 - Acids, Bases and Salts (1)Document6 pages2 - Acids, Bases and Salts (1)HONEY YOYONo ratings yet
- GRADE 10 Chemical ReactionsDocument17 pagesGRADE 10 Chemical ReactionsDanny BlessyNo ratings yet
- Social ScienceDocument18 pagesSocial Sciencemonika.yogaNo ratings yet
- Chemical Reactions and Equations NotesDocument13 pagesChemical Reactions and Equations NotesJayanthiNo ratings yet
- Chemical Reactions & Equations: Type (I) : Very Short Answer Type Questions: (01 Mark Each) 1Document12 pagesChemical Reactions & Equations: Type (I) : Very Short Answer Type Questions: (01 Mark Each) 1Prathmesh NamanNo ratings yet
- Chem Activity BK 1B (Answers)Document40 pagesChem Activity BK 1B (Answers)trumpNo ratings yet
- Acid Bases and SaltsDocument7 pagesAcid Bases and SaltsSubhash suhasariaNo ratings yet
- Summer Vacation Holiday Homework2023-24 Science 10THDocument15 pagesSummer Vacation Holiday Homework2023-24 Science 10THAstitva SinghNo ratings yet
- NCERT Exemplar For Class 10 Science Chapter 1Document34 pagesNCERT Exemplar For Class 10 Science Chapter 1Saisha AroraNo ratings yet
- ICSE-Class 9 - Chemistry-Chapter-06-Chemical Reaction-QnADocument13 pagesICSE-Class 9 - Chemistry-Chapter-06-Chemical Reaction-QnAAmmolh MahajanNo ratings yet
- Solutions - Chemical ReactionsDocument8 pagesSolutions - Chemical ReactionschetanNo ratings yet
- Chemistry Form 4Document3 pagesChemistry Form 4Nora MnNo ratings yet
- Chemical Reactions - Notes & Q - ADocument21 pagesChemical Reactions - Notes & Q - AYOGESHNo ratings yet
- Question BankDocument180 pagesQuestion BankLVAM GAMINGNo ratings yet
- Chemical Reactions and EquationsDocument45 pagesChemical Reactions and EquationsSaloni ChaudharyNo ratings yet
- Precipitation ReactionsDocument19 pagesPrecipitation ReactionsShada SalloumNo ratings yet
- Question 1Document22 pagesQuestion 1rajindia698No ratings yet
- Cbse Test Paper-01 01 Chemical Reactions and EquationsDocument7 pagesCbse Test Paper-01 01 Chemical Reactions and Equationsashish.raj242008No ratings yet
- Chapter 01 Chemical Reactions and Equations Test Paper 01Document7 pagesChapter 01 Chemical Reactions and Equations Test Paper 01laurelmatthewlNo ratings yet
- Chemical Reactions and Equations-Question BankDocument23 pagesChemical Reactions and Equations-Question Bankharrissraghavv18No ratings yet
- Cbse Test Paper-01 CLASS - X Science (Chemical Reactions and Equations)Document4 pagesCbse Test Paper-01 CLASS - X Science (Chemical Reactions and Equations)Neerraj YadavNo ratings yet
- Science - Test Paper - X - CH 1Document4 pagesScience - Test Paper - X - CH 1Víshál RánáNo ratings yet
- 3 Nazism & The Rise of HitlerDocument14 pages3 Nazism & The Rise of Hitlersciencee2009No ratings yet
- 4 Forest Society and ColonialismDocument11 pages4 Forest Society and Colonialismsciencee2009No ratings yet
- 2 Socialism in Europe and The Russian RevolutionDocument17 pages2 Socialism in Europe and The Russian Revolutionsciencee2009No ratings yet
- 1 The French RevolutionDocument19 pages1 The French Revolutionsciencee2009No ratings yet
- 5 Pastoralists in The Modern WorldDocument11 pages5 Pastoralists in The Modern Worldsciencee2009No ratings yet
- 4 Structure-Of-The-AtomDocument9 pages4 Structure-Of-The-Atomsciencee2009No ratings yet
- Observation: Activity 3.8 Class 10 ScienceDocument1 pageObservation: Activity 3.8 Class 10 Sciencesciencee2009No ratings yet
- 3 Atoms-And-MoleculesDocument7 pages3 Atoms-And-Moleculessciencee2009No ratings yet
- 5 The-Fundamental-Unit-Of-LifeDocument5 pages5 The-Fundamental-Unit-Of-Lifesciencee2009No ratings yet
- 1 Matter-In-Our-SurroundingsDocument5 pages1 Matter-In-Our-Surroundingssciencee2009No ratings yet
- Activity-Put About 2 G Soil in A Test Tube and Add 5 ML Water To ItDocument1 pageActivity-Put About 2 G Soil in A Test Tube and Add 5 ML Water To Itsciencee2009No ratings yet
- Activity 2.5 Explanation: How Do Metal Carbonates and Metal Hydrogencarbonates React With Acids?Document1 pageActivity 2.5 Explanation: How Do Metal Carbonates and Metal Hydrogencarbonates React With Acids?sciencee2009No ratings yet
- Activity-Write The Formulae of The Salts Given Below. Potassium Sulphate, Sodium Sulphate, CalciumDocument1 pageActivity-Write The Formulae of The Salts Given Below. Potassium Sulphate, Sodium Sulphate, Calciumsciencee2009No ratings yet
- Explain Activity 1.4 Class 10 NCERT Science: Name The Product Formed When Calcium Oxide Reacts With Water?Document2 pagesExplain Activity 1.4 Class 10 NCERT Science: Name The Product Formed When Calcium Oxide Reacts With Water?sciencee2009No ratings yet
- Activity 2.6 Explanation: How Do Acids and Bases React With Each Other?Document1 pageActivity 2.6 Explanation: How Do Acids and Bases React With Each Other?sciencee2009No ratings yet
- Observation ConclusionDocument2 pagesObservation Conclusionsciencee2009No ratings yet
- Explain Activity 1.8 Class 10 NCERT Science: Caution: This Activity Needs The Teacher's Assistance. AimDocument2 pagesExplain Activity 1.8 Class 10 NCERT Science: Caution: This Activity Needs The Teacher's Assistance. Aimsciencee2009No ratings yet
- Filler Wires LeafletDocument2 pagesFiller Wires LeafletAshok RajNo ratings yet
- Chemical NomenclatureDocument3 pagesChemical NomenclatureDane デーン MalilayNo ratings yet
- Organic Chemistry 2Document5 pagesOrganic Chemistry 2ibdpNo ratings yet
- Lab Values Tips and TricksDocument2 pagesLab Values Tips and TricksEliezer NuenayNo ratings yet
- Licensed To Arun Kumar Das: Is 228 (Part 1) : 1987 Methods For Chemical Analysis ofDocument5 pagesLicensed To Arun Kumar Das: Is 228 (Part 1) : 1987 Methods For Chemical Analysis ofIndira MukherjeeNo ratings yet
- Advanced-Chem Q1 LP7Document6 pagesAdvanced-Chem Q1 LP7Francesca BuenoNo ratings yet
- Issuer & Norm Modality / Abbreviation Metal Coat Thickness in MDocument10 pagesIssuer & Norm Modality / Abbreviation Metal Coat Thickness in MBartek HajaNo ratings yet
- Uses of IronDocument2 pagesUses of IronmaterialmindedNo ratings yet
- Kjeldahl Method For Determining NitrogenDocument3 pagesKjeldahl Method For Determining Nitrogendenytrung100% (1)
- MODULE 6 INCHEM PDF Chemical Calculations and EquationsDocument51 pagesMODULE 6 INCHEM PDF Chemical Calculations and EquationsFrancisDanielRoaNo ratings yet
- Module 2Document19 pagesModule 2ManjunathNo ratings yet
- PREPARATION OF POTASSIUM ALUMINUM SULFATE, KAl (SO4) 2 - 12H2O (Alum)Document5 pagesPREPARATION OF POTASSIUM ALUMINUM SULFATE, KAl (SO4) 2 - 12H2O (Alum)vinsmoke1No ratings yet
- Experiment 6 (Elemental Analysis)Document1 pageExperiment 6 (Elemental Analysis)Lemuel VillanuevaNo ratings yet
- Revised Products Effective April 2008Document65 pagesRevised Products Effective April 2008EslamNo ratings yet
- Electroplating of Chromium Coatings From CR (III) - Based Electrolytes Containing Water Soluble PolymerDocument10 pagesElectroplating of Chromium Coatings From CR (III) - Based Electrolytes Containing Water Soluble Polymertonny356No ratings yet
- Sodium Iodide (131I) Solution PDFDocument2 pagesSodium Iodide (131I) Solution PDFLaurentiu DinuNo ratings yet
- Erw Pipe Manufacturing Process PDFDocument2 pagesErw Pipe Manufacturing Process PDFJabulaniNo ratings yet
- 17.salt Aluminium Sulphate 3Document3 pages17.salt Aluminium Sulphate 3Sarthika GaulkarNo ratings yet
- CBSE Class 10 Chemistry Worksheet - Chemical Reactions and EquationsDocument3 pagesCBSE Class 10 Chemistry Worksheet - Chemical Reactions and EquationsLushila Minj100% (1)
- BomDocument3 pagesBomsandeepNo ratings yet
- Exp 5 Flame Tests and Electron ConfigurationDocument7 pagesExp 5 Flame Tests and Electron ConfigurationJean OlbesNo ratings yet
- Dissimilar Metal Weldingv2 HERBST PDFDocument23 pagesDissimilar Metal Weldingv2 HERBST PDFAnonymous q2HC0zyfa2No ratings yet
- IMCO Catalog 2007Document106 pagesIMCO Catalog 2007shaheenkhan2510No ratings yet
- Reach & Rohs Material Declaration of Conformity: The Harris Products GroupDocument2 pagesReach & Rohs Material Declaration of Conformity: The Harris Products GroupTowfiq AhmedNo ratings yet
- June 2019 (v1) QP - Paper 4 CIE Chemistry IGCSEDocument16 pagesJune 2019 (v1) QP - Paper 4 CIE Chemistry IGCSEdovoo lolNo ratings yet
- 1 Hydrogen Peroxide Decomposes To Form Water and Oxygen. This Reaction Is Catalysed byDocument9 pages1 Hydrogen Peroxide Decomposes To Form Water and Oxygen. This Reaction Is Catalysed byKelvin DenhereNo ratings yet
- SdfasdfDocument5 pagesSdfasdfMarman Fabro Anga-AnganNo ratings yet
- 3EChem PRACTICE PAPER 2Document17 pages3EChem PRACTICE PAPER 2Alley EioNo ratings yet
- CPDScardno 044-0141Document3 pagesCPDScardno 044-0141murie dwiyanitiNo ratings yet