Professional Documents
Culture Documents
1 of 20 1 of 36: © Boardworks LTD 2004 © Boardworks LTD 2005
Uploaded by
MindOfPrince0 ratings0% found this document useful (0 votes)
16 views24 pagesOriginal Title
Spotting_Patterns
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
16 views24 pages1 of 20 1 of 36: © Boardworks LTD 2004 © Boardworks LTD 2005
Uploaded by
MindOfPrinceCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 24
1 of 36
20 © Boardworks Ltd 2005
2004
Spotting patterns
• L.O.: • H.W:
1- To identify the physical • 1-Name the least
and the chemical properties. reactive metal in group
1
2- Spotting patterns and
trends ascross periods and • 2- How many outer
down groups. shell electrons in:
3- Electron arrangement in • Group 1
shells. • Group 2
2- Keywords: • Draw an atomic
• properties. diagram for Mg.
• shells
•
Periodic Law
The periodic recurrence of elements with similar
physical and chemical properties, when the
elements are listed in order of increasing atomic
number, results directly from the periodic
recurrence of similar electronic configurations in
the outer shells of respective atoms
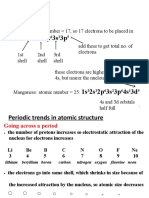
Arrangement of electrons
Valence electrons
• Valence electrons are the electrons in the outermost electron shell of
an element. Sometimes, it is also regarded as the basis of Modern
Periodic Table. In a period, the number of valence electrons increases
as we move from left to right side. However, in a group this periodic
trend is constant, that is the number of valence electrons remains the
same.
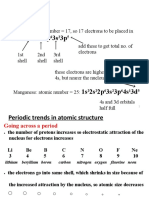
The trend in boiling point in periods 2&3 elements
• A graph to show how atomic number affects the atomic mass- this data
will allow the chemist to predict the state of the element at room
temperature
• As you move from the top to the bottom of the periodic
table, you’ll find a rough correlation exists between the
atomic mass of elements and their boiling points. Lighter
elements such as hydrogen and helium tend to have very low
boiling points, and elements with greater atomic mass boil at
higher temperatures. The atomic mass affects the forces
between atoms, which in turn determine boiling points.
• London Forces
• Attractive forces between atoms in a substance affect its boiling point. The stronger the force, the harder it is to “pry” atoms
apart from each other and turn them into a gas; elements with high boiling points require more heat energy to separate the
atoms. Chemists call this attraction the London Dispersion force; it is produced by the random motions of electrons in atoms,
where one side of an atom is momentarily more negative than the other, attracting other atoms with the opposite electric
charge. Although London forces are weak compared to other bonding forces, they strongly influence melting and boiling points.
• Atomic Mass
• Most periodic tables list the atomic mass and atomic number for every element. The atomic mass is the number of protons and
neutrons added together. As elements have isotopes having different numbers of neutrons, the periodic table gives an average
mass. The atomic number is simply the number of protons, which is a constant for each element. The table starts with the lowest
atomic numbers at the top left and has the highest at the bottom right. Atomic masses increase as atomic numbers do, so the
two are strongly linked.
• Periodic Column Trend
• As you progress downwards in the periodic table along a given column, the atomic mass tends to increase along with the boiling
point. For example, in the column for noble gases, helium, at the very top, has a boiling point of minus 269 degrees Celsius
(minus 452 degrees Fahrenheit). Progressing towards the bottom, the element krypton boils at minus 152 degrees Celsius (minus
242 degrees Fahrenheit). Radon, at the bottom of the column, boils at minus 62 degrees Celsius (minus 79 degrees Fahrenheit) --
warmer by 207 degrees Celsius (404 degrees Fahrenheit).
• Exceptions to the Trend
• You won’t find an absolute correlation between atomic mass and boiling point; several exceptions contradict the rule. For
example, carbon, which has a relatively modest atomic mass of about 12, also has the highest boiling point of any element --
3,825 degrees Celsius (6,917 degrees Fahrenheit) -- due to the strong covalent bonds between atoms. Beryllium also has an
exceptionally high boiling point at 2,471 degrees Celsius (4,480 degrees Fahrenheit) with a low atomic mass of 9. Another
exception is the metal mercury, with a relatively low boiling point of 356.73 degrees Celsius (674.11 degrees Fahrenheit) despite
a hefty atomic mass of 200.6.
You might also like
- 1 of 20 1 of 36: © Boardworks LTD 2004 © Boardworks LTD 2005Document19 pages1 of 20 1 of 36: © Boardworks LTD 2004 © Boardworks LTD 2005MindOfPrinceNo ratings yet
- Ib Chemistry: Topic 3 PeriodicityDocument90 pagesIb Chemistry: Topic 3 Periodicitynoob masterNo ratings yet
- Ib PPT 3 SL PDFDocument24 pagesIb PPT 3 SL PDFzarna nirmal rawalNo ratings yet
- Trends - Periodic TableDocument34 pagesTrends - Periodic Tableaaahluma.gxeesiNo ratings yet
- Atomic StructureDocument32 pagesAtomic StructurePaul AckermannNo ratings yet
- Periodic Properties Chemistry Class 11Document32 pagesPeriodic Properties Chemistry Class 11Ravinder singhNo ratings yet
- Notes - Periodic Classification of Elements - C-XDocument4 pagesNotes - Periodic Classification of Elements - C-Xpratishtha MishraNo ratings yet
- Lecture 2Document10 pagesLecture 2siphosakhemdunge114No ratings yet
- 20 Page GCSE To AS Transition BookletDocument20 pages20 Page GCSE To AS Transition BookletHanaNo ratings yet
- Group 3: Periodic Relationship: Wendell Bandiola (THE PERIODIC TABLE)Document66 pagesGroup 3: Periodic Relationship: Wendell Bandiola (THE PERIODIC TABLE)Kristel Ann LaudeNo ratings yet
- Bai GiangDocument104 pagesBai GiangminhyNo ratings yet
- 1s 2s 2p 3s 3p: ExamplesDocument104 pages1s 2s 2p 3s 3p: ExamplesminhyNo ratings yet
- ChemistryDocument65 pagesChemistryJohnpaul KondoNo ratings yet
- Periodic Properties of The ElementsDocument49 pagesPeriodic Properties of The ElementsSatish BabuNo ratings yet
- Chemistry 3U Exam ReviewDocument19 pagesChemistry 3U Exam ReviewHannah PilonNo ratings yet
- Topic 1 An Overview of The Periodic TableDocument37 pagesTopic 1 An Overview of The Periodic TableHafizh PpNo ratings yet
- Periodic Table: Oakland Schools Chemistry Resource UnitDocument42 pagesPeriodic Table: Oakland Schools Chemistry Resource UnitAnum TauqirNo ratings yet
- Atoms, Electrons & Energy Levels: Electrons Are The Bonds That Hold The World Together!Document41 pagesAtoms, Electrons & Energy Levels: Electrons Are The Bonds That Hold The World Together!cmillica1176100% (3)
- The Periodic Trends Updated 2023Document58 pagesThe Periodic Trends Updated 2023stutireddy1912No ratings yet
- Periodic Table-WPS OfficeDocument30 pagesPeriodic Table-WPS Officenabeel0% (1)
- Revision Guide For GCSE Science ChemistryDocument9 pagesRevision Guide For GCSE Science Chemistryjenny10040% (1)
- K03398 - 20210309182549 - SKT3033 - Topic 1 An Overview of The Periodic TableDocument35 pagesK03398 - 20210309182549 - SKT3033 - Topic 1 An Overview of The Periodic Tableliana aliaNo ratings yet
- Examkrackers General Chemistry NotesDocument16 pagesExamkrackers General Chemistry NotesddNo ratings yet
- LP 6 Inorganic Chemistry With TemplateDocument10 pagesLP 6 Inorganic Chemistry With TemplateJOHNERROL CARCELLARNo ratings yet
- 1-Atoms and Molecules - 2022Document50 pages1-Atoms and Molecules - 2022riva rizkianaNo ratings yet
- Year 10 ChemistryDocument26 pagesYear 10 ChemistryBigDaddy GNo ratings yet
- AOS1: The Periodic Table: Atomic Theory RevisionDocument6 pagesAOS1: The Periodic Table: Atomic Theory RevisionNicola NguyenNo ratings yet
- Lecture 3+4: Periodic Properties Off The ElementsDocument34 pagesLecture 3+4: Periodic Properties Off The ElementsHIEP PHAM HOANGNo ratings yet
- Chemical BondingDocument25 pagesChemical Bondingmaxwell amponsahNo ratings yet
- Periodic Table of ElementsDocument76 pagesPeriodic Table of ElementsNursaiyidah RoniNo ratings yet
- Periodic Trends CompleteDocument51 pagesPeriodic Trends CompleteZal Fildan DuomaNo ratings yet
- 3A The Periodic TableDocument7 pages3A The Periodic TableMohammed TarekNo ratings yet
- Periodic Classification of Elements Xerox 2020Document7 pagesPeriodic Classification of Elements Xerox 2020irehan.saiyedNo ratings yet
- Ib Chemistry: Topic 3 PeriodicityDocument60 pagesIb Chemistry: Topic 3 PeriodicityMichellycia AgathaNo ratings yet
- CH4701/4001 2019 Lecture 16 and 17 Trends in The Periodic TableDocument52 pagesCH4701/4001 2019 Lecture 16 and 17 Trends in The Periodic TableJason ZhangNo ratings yet
- Periodic Table NotesDocument34 pagesPeriodic Table NotesMiraNo ratings yet
- F321 PeriodicityDocument3 pagesF321 PeriodicityDoc_CrocNo ratings yet
- Pertemuan 2 - Kimia - DosenDocument49 pagesPertemuan 2 - Kimia - Dosenraaflie caesarNo ratings yet
- IGCSE ChemistryDocument12 pagesIGCSE Chemistryc21fw.csyNo ratings yet
- Chemistry Sec-ADocument51 pagesChemistry Sec-ASajid AkandNo ratings yet
- Namma Kalvi 12th Chemistry PowerPoint Presentation Material EM 219360Document99 pagesNamma Kalvi 12th Chemistry PowerPoint Presentation Material EM 219360Anant Mathew SibyNo ratings yet
- Chapter 2 Material Structure and BondingDocument31 pagesChapter 2 Material Structure and BondingAziziell PDracingNo ratings yet
- Chemistry Review SheetDocument9 pagesChemistry Review SheetEric ThatAsian Yao100% (1)
- Chemistry Edexcel Unit 2 AsDocument18 pagesChemistry Edexcel Unit 2 Asminayoki0% (1)
- Chemistry SS2 EditedDocument150 pagesChemistry SS2 EditedSamuel BiyamaNo ratings yet
- Physical Properties of Oil and ChemicalDocument101 pagesPhysical Properties of Oil and ChemicalD kuiNo ratings yet
- Bonding: General ConceptsDocument113 pagesBonding: General ConceptsRhythm's PathakNo ratings yet
- Atomic and Crystal Structure of MaterialsDocument52 pagesAtomic and Crystal Structure of Materialscharles makasabiNo ratings yet
- 24TH fEB 234Document42 pages24TH fEB 234hamzaNo ratings yet
- Structure & Bonding: Types of BondDocument13 pagesStructure & Bonding: Types of BondWazeem MohammedNo ratings yet
- Structure of MatterDocument54 pagesStructure of MatterMustafa SaßerNo ratings yet
- CHM111E 1.1.1 - Periodic Table and Its Trends PDFDocument43 pagesCHM111E 1.1.1 - Periodic Table and Its Trends PDFanton petrovNo ratings yet
- AP Chemistry Review PacketDocument32 pagesAP Chemistry Review Packetlycheejello100% (3)
- Atoms and The Periodic Tabl0Document64 pagesAtoms and The Periodic Tabl0Ana Lucía TrejosNo ratings yet
- Chapter 2 For OnlineDocument35 pagesChapter 2 For OnlineKarina Roxana Marquez TumeNo ratings yet
- History of The PeriodicTableDocument50 pagesHistory of The PeriodicTablePscf CarmonaNo ratings yet
- IMFA and Chemical BondingDocument137 pagesIMFA and Chemical BondingEnna SertNo ratings yet
- Practice Makes Perfect in Chemistry: The Periodic Table with AnswersFrom EverandPractice Makes Perfect in Chemistry: The Periodic Table with AnswersRating: 5 out of 5 stars5/5 (1)
- Reactivity SeriesDocument25 pagesReactivity SeriesMindOfPrinceNo ratings yet
- Metals and Non-Metals 1Document32 pagesMetals and Non-Metals 1MindOfPrinceNo ratings yet
- Food Digestion To PrintDocument44 pagesFood Digestion To PrintMindOfPrinceNo ratings yet
- Atom Element and Atomic StructrureDocument48 pagesAtom Element and Atomic StructrureMindOfPrinceNo ratings yet
- Multiplication 1Document45 pagesMultiplication 1OnucaNo ratings yet
- Aits 2223 Ot Jeem TD OfflineDocument20 pagesAits 2223 Ot Jeem TD OfflineSuvrajyoti TaraphdarNo ratings yet
- Acsaelm 1c00281Document8 pagesAcsaelm 1c00281Rishi MaitiNo ratings yet
- DPP 1 NucleiDocument3 pagesDPP 1 NucleiSidhu MoosewaalaNo ratings yet
- AnisotropyDocument6 pagesAnisotropyDondieNo ratings yet
- 0625 - Y03 - SQ - 10 Atomic PhysicsDocument16 pages0625 - Y03 - SQ - 10 Atomic Physicswafaul_athiyyahNo ratings yet
- CH (13) NucleiDocument24 pagesCH (13) Nucleikhurafati.amratanshNo ratings yet
- Diagnostic Test in Physical ScienceDocument4 pagesDiagnostic Test in Physical ScienceMELTON MERZANo ratings yet
- Radiography TestingDocument23 pagesRadiography TestingRamesh RNo ratings yet
- 02-Electron, Photon, PEE-sub-1, 2, 3 and 4Document23 pages02-Electron, Photon, PEE-sub-1, 2, 3 and 4Tejas pawarNo ratings yet
- WPH05 01 MSC 20190307Document16 pagesWPH05 01 MSC 20190307Abdi KhadarNo ratings yet
- Radiation PhysicsDocument307 pagesRadiation PhysicsHarley Alejo MNo ratings yet
- Tritium (Hydrogen-3) : What Is It?Document2 pagesTritium (Hydrogen-3) : What Is It?Amit AcharyaNo ratings yet
- NEET - 2022: ChemistryDocument175 pagesNEET - 2022: ChemistryAman B NairNo ratings yet
- Organic Chemistry With AnswersDocument3 pagesOrganic Chemistry With AnswersAhmed HashkarNo ratings yet
- Chemistry 0620 NotesDocument192 pagesChemistry 0620 Notesmohammed mahdyNo ratings yet
- QM-2 Solved ProblemsDocument17 pagesQM-2 Solved ProblemsHenock TadeleNo ratings yet
- Ncert ch2 Chemistry Class 11Document44 pagesNcert ch2 Chemistry Class 11Karan ManglaNo ratings yet
- Green Chemistry: Tuning The Coordination Number of Fe Single Atoms For The e Cient Reduction of CODocument8 pagesGreen Chemistry: Tuning The Coordination Number of Fe Single Atoms For The e Cient Reduction of COcrystalquasiNo ratings yet
- Cambridge IGCSE: Co-Ordinated Sciences 0654/43Document32 pagesCambridge IGCSE: Co-Ordinated Sciences 0654/43Raghav SharmaNo ratings yet
- Nuclear Physics Lab 8Document5 pagesNuclear Physics Lab 8tashy richardsNo ratings yet
- Atomic Structure and Bonding - AnswerDocument20 pagesAtomic Structure and Bonding - Answer6brk8sjszqNo ratings yet
- ANAPH121 Part 2 Basic ChemistryDocument62 pagesANAPH121 Part 2 Basic ChemistryAB AlmazoraNo ratings yet
- Periodic Table WorksheetDocument23 pagesPeriodic Table Worksheetlakshmi ghayathri N.M.No ratings yet
- Nuclear Thermal Hydraulics (2016)Document464 pagesNuclear Thermal Hydraulics (2016)Mrredstone 53No ratings yet
- Photoelectric Effect1Document42 pagesPhotoelectric Effect1Pearl Shane DomingoNo ratings yet
- 7 Lab Assistant Final SyllabusDocument3 pages7 Lab Assistant Final Syllabuspokhrelsuman165No ratings yet
- Moving Charge in A Magnetic FieldDocument3 pagesMoving Charge in A Magnetic FieldEntertainmentNo ratings yet
- 05-07-20 - SR - Super 60 - (In Com) - Jee-Adv2018-P1 - UTA-14 - QPDocument17 pages05-07-20 - SR - Super 60 - (In Com) - Jee-Adv2018-P1 - UTA-14 - QPIITIANNo ratings yet
- Structure of Atom Shobhit NirwanDocument15 pagesStructure of Atom Shobhit NirwanBhavya Goyal XI Non med91% (11)