Professional Documents
Culture Documents
Endocrine System Assesment
Uploaded by
Yudiatma100%(1)100% found this document useful (1 vote)
16 views51 pagesOriginal Title
ENDOCRINE SYSTEM ASSESMENT
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
100%(1)100% found this document useful (1 vote)
16 views51 pagesEndocrine System Assesment
Uploaded by
YudiatmaCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 51
ENDOCRINE SYSTEM ASSESMENT
Dr. Ns. Eko Winarto, M.Kep., Sp.MB
(Diklat RSUD Banyumas)
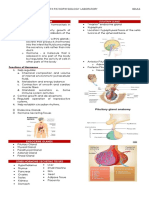
REVIEW
• Starling coined the term hormone to describe secretin, a
substance secreted by the small intestine into the blood stream
to stimulate pancreatic secretion.
• Starling considered the endocrine and nervous systems as two
distinct mechanisms for coordination and control of organ
function.
• the characterization of many hormones secreted into the blood
stream from discrete glands or other organs.
BACKGROUND
• Hormones can be defined as chemical signals secreted into the
blood stream that act on distant tissues, usually in a regulatory
fashion. Hormonal signaling represents a special case of the
more general process of signaling between cells.
• Signals from one cell to adjacent cells, so-called paracrine
signals, often trigger cellular responses that use the same
molecular pathways used by hormonal signals.
• Target cells respond similarly to signals that reach them from
the blood stream (hormones) or from the cell next door
(paracrine factors);
• Testosterone is secreted into the blood stream but also acts
locally in the testes to control spermatogenesis.
• Insulin-like growth factor I is a hormone secreted into the blood
stream from the liver and other tissues, but it is also a paracrine
factor made locally in most tissues to control cell proliferation.
• One receptor can mediate the actions of a hormone, such as
parathyroid hormone, and of a paracrine factor, such
parathyroid hormonerelated protein.
• Hormone formation may occur either in localized collections of
specific cells, in the endocrine glands, or in cells that have
additional roles. Many protein hormones, such as growth
hormone, parathyroid hormone, prolactin, insulin, and glucagon,
are produced in dedicated cells by standard protein synthetic
mechanisms common to all cells. These secretory cells usually
contain specialized secretory granules designed to store large
amounts of hormone and to release the hormone in response to
specific signals.
• Formation of small hormone molecules initiates with commonly
found precursors, usually in specific glands such as the
adrenals, gonads, or thyroid. In the case of the steroid
hormones, the precursor is cholesterol, which is modified by
various hydroxylations, methylations, and demethylations to
form the glucocorticoids, androgens, and estrogens, and their
biologically active derivatives.
• In contrast, the precursor of vitamin D, 7-dehydrocholesterol, is
produced in skin keratinocytes, again from cholesterol, by a
photochemical reaction. Leptin, which regulates appetite and
energy expenditure, is formed in adipocytes, thus providing a
specific signal reflecting the organism's nutritional state to the
central nervous system.
• Thyroid hormone synthesis occurs via a unique pathway. The
thyroid cell synthesizes a 660,000-kd homodimer, thyroglobulin,
which is then iodinated at specific iodotyrosines. Certain of
these "couple" to form the iodothyronine molecule within
thyroglobulin, which is then stored in the lumen of the thyroid
follicle. In order for this to occur, the thyroid cell must
concentrate the trace quantities of iodide from the blood and
oxidize it via a specific peroxidase. Release of thyroxine (T 4 )
from the thyroglobulin requires its phagocytosis and cathepsin-
catalyzed digestion by the same cells.
• Hormones are synthesized in response to biochemical signals
generated by various modulating systems. Many of these
systems are specific to the effects of the hormone product; for
example, parathyroid hormone synthesis is regulated by the
concentration of ionized calcium, whereas gonadal, adrenal, and
thyroid hormone synthesis is achieved by the hormonostatic
function of the hypothalamicpituitary axis.
• Cells in the hypothalamus and pituitary monitor the circulating
hormone concentration and secrete trophic hormones that
activate specific pathways for hormone synthesis and release.
Typical examples are luteinizing (LH) follicle-stimulating (FSH),
thyroid-stimulating (TSH), and adrenocorticotrophic (ACTH)
hormones.
• These trophic hormones increase rates of hormone synthesis
and secretion and also may induce target cell division, thus
causing enlargement of the various target glands. For example,
in hypothyroid individuals living in iodine-deficient areas of the
world, TSH secretion causes a marked hyperplasia of thyroid
cells. In such regions, the thyroid gland may be 20- to 50-fold its
normal size.
• Adrenal hyperplasia occurs in patients with genetic deficiencies
in cortisol formation. Hypertrophy and hyperplasia of parathyroid
cells, in this case initiated by an intrinsic response to the stress
of hypocalcemia, occur in patients with renal insufficiency or
calcium malabsorption.
• Hormones are synthesized as required on a daily, hourly, or
minute-to-minute basis with minimal storage, but there are
significant exceptions. One such exception is the thyroid gland,
which contains enough stored hormone to last for about two
months. This permits a constant supply of this hormone despite
significant variations in the availability of iodine. If iodine
deficiency is prolonged, however, the normal reservoirs of
thyroxine can be depleted.
• The various feedback signaling systems exemplified above provide the hormonal
homeostasis characteristic of virtually all endocrine systems. Regulation may
include the central nervous system or local signal recognition mechanisms in the
glandular cells, such as the calcium-sensing receptor of the parathyroid cell.
Superimposed, centrally programmed increases and decreases in hormone
secretion or activation through neuroendocrine pathways also occur.
• the circadian variation in the secretion of ACTH directing the synthesis and
release of cortisol. The monthly menstrual cycle exemplifies a system with much
longer periodicity that requires a complex synergism between central and
peripheral axes of the endocrine glands. Disruption of hormonal homeostasis
due to glandular or central regulatory system dysfunction has both clinical and
laboratory consequences. Recognition and correction of these are the essence
of clinical endocrinology.
TARGET CELLS AS ACTIVE PARTICIPANTS
• Hormones determine cellular target actions by binding with high
specificity to receptor proteins. Whether a peripheral cell is
hormonally responsive depends to a large extent on the
presence and function of specific and selective hormone
receptors. Receptor expression thus determines which cells will
respond, as well as the nature of the intracellular effector
pathways activated by the hormone signal. Receptor proteins
may be localized to the cell membrane, cytoplasm, and nucleus.
Broadly, polypeptide hormone receptors are cell-membrane
associated, whereas soluble intracellular proteins selectively
bind to steroid hormones.
• Membrane-associated receptor proteins usually consist of
extracellular sequences that recognize and bind ligand,
transmembrane anchoring hydrophobic sequences, and
intracellular sequences, which initiate intracellular signaling.
• Intracellular signaling is mediated by soluble second
messengers (e.g., cyclic AMP) or by activation of intracellular
signaling molecules (e.g., signal transduces and activates of
transcription [STAT] proteins). Receptor-dependent activation of
heterotrimeric G-proteins, comprising and subunits, may either
induce or suppress effector enzymes or ion channels.
HORMONE MEASUREMENT
• Endocrine function can be assessed by measuring levels of basal circulating
hormone, evoked or suppressed hormone, or hormone-binding proteins.
Alternatively, peripheral hormone receptor function can be assessed. Meaningful
strategies for timing hormonal measurements vary from system to system. In some
cases, circulating hormone concentrations can be measured in randomly collected
serum samples. This measurement, when standardized for fasting, environmental
stress, age, and gender, is reflective of true hormone concentrations only when
levels do not fluctuate appreciably. For example, thyroid hormone, prolactin, and IGF-
I levels can be accurately assessed in fasting morning serum samples. On the other
hand, when hormone secretion is clearly episodic, timed samples may be required
over a defined time course to reflect hormone bioavailability. Thus, early morning and
late evening cortisol measurements are most appropriate. Although 24-hour sampling
for GH measurements, with samples collected every 2, 10, or 20 minutes, are
expensive and cumbersome, they may yield valuable diagnostic information. Random
sampling may also reflect secretion peaks or nadirs, thus confounding adequate
interpretation of results.
ENDOCRINE DISEASES
• Endocrine diseases fall into four broad categories: (1) hormone
overproduction; (2) hormone underproduction; (3) altered tissue
responses to hormones; and (4) tumors of endocrine glands.
HORMONE OVERPRODUCTIO
• Hormones are secreted in increased amounts because of genetic
abnormalities that cause abnormal regulation of hormone synthesis or
release.
• Diseases of hormone overproduction are associated with an increase in the
total number of hormone-producing cells. For example, the hyperthyroidism
of Graves' disease, in which antibodies mimic TSH and activate the TSH
receptors on thyroid cells, is associated with dramatic increase in thyroid cell
proliferation, as well as with increased synthesis and release of thyroid
hormone from each thyroid cell.
• Endocrine tumors are not polyclonal expansions, however, but instead
represent monoclonal expansions of one mutated cell. These mutations lead
to an increase in proliferation and/or survival of the mutant cells.
HORMONE UNDERPRODUCTION
• Underproduction of hormone can result from a wide variety of processes,
ranging from surgical removal of parathyroid glands during neck surgery, to
tuberculous destruction of adrenal glands, or to iron deposition in -cells in
hemochromatosis.
• A frequent cause of destruction of hormone-producing cells is autoimmunity.
Autoimmune destruction of beta cells in type 1 diabetes mellitus and
autoimmune destruction of thyroid cells in Hashimoto's thyroiditis are two of
the most common disorders treated by endocrinologists.
• More uncommonly, a host of genetic abnormalities can also lead to
decreased hormone production. These disorders can result from abnormal
development of hormone-producing cells (e.g., hypogonadotrophic
hypogonadism caused by KAL gene mutations), from abnormal synthesis of
hormones (e.g., deletion of the growth hormone gene), or from abnormal
regulation of hormone secretion (e.g., the hypoparathyroidism associated
with activating mutations of the parathyroid cell's calcium-sensing receptor).
ALTERED TISSUE RESPONSES
• Resistance to hormones can be caused by a variety of genetic disorders.
Examples include mutations in the growth hormone receptor in Laron
dwarfism and mutations in the G gene in the hypoparathyroidism of
pseudohypoparathyroidism, type 1a.
• The insulin resistance in muscle and liver central to the etiology of type 2
diabetes mellitus appears to be polygenic in origin. Type 2 diabetes is also
an example of a disease in which end organ insensitivity is worsened by
signals from other organs, in this case by signals originating in fat cells.
• The target organ of hormone action is more directly abnormal, as in the
parathyroid hormone (PTH) resistance of renal failure. Increased end organ
function can be caused by mutations in signal reception and propagation.
TUMORS OF ENDOCRINE GLANDS
• Tumors of endocrine glands, as noted above, often result in
hormone overproduction. Some tumors of endocrine glands
produce little if any hormone but cause disease by their local
compressive symptoms or by metastatic spread. Examples
include so-called nonfunctioning pituitary tumors, which are
usually benign but can cause a variety of symptoms due to
compression on adjacent structures, and thyroid cancer, which
can spread throughout he body without causing hyperthyroidism
ASSESMENT
GENERAL CONSIDERATIONS
• Many features of being an endocrine patient are common to all
experiences of illness. Although generations of medical students
have described new patients as being "in no acute distress,"
most patients are, in fact, worried and anxious. A few minutes
spent in getting to know the patient can pay enormous dividends
in the accuracy of the history obtained and in setting the stage
for further cooperation with testing and treatment.
• You are interested in the patient as a person and not just as a
disease.
DISCOVERY THROUGH SCREENING
• Numerous special features of endocrine disease make patient
presentation quite different from that seen in general medicine.
One is the discovery of abnormality through screening of
asymptomatic individuals, for example, a high serum calcium
level discovered through multiphasic screening or a high blood
glucose level discovered in a shopping mall kiosk.
• The very absence of symptoms lends an unreality to the
moment and should become an explicit topic of the patient-
doctor interaction. In this circumstance, it is worth emphasizing
the value of early discovery and prevention of greater morbidity
QUANTITATIVE RATHER THAN QUALITATIVE
ABNORMALITIES
• A second special feature of endocrine disorders is that they are
all quantitative, rather than qualitative, departures from normal.
No endocrine disorder is due to a novel hormone. Everyone has
cortisol circulating as a determining feature of his or her life.
Hypercorticism and adrenal insufficiency represent just more or
less of the hormone. Similarly, all hormones found in excess or
in deficiency in disease are physiologic determinants of stature,
weight, complexion, hairiness, temperament, and behavior.
OVERLAP WITH OTHER DISEASES
• The symptoms of endocrine disorders overlap a great range of
normal characteristics, including body contour, facial
configurations, weight distributions, skin and hair coloring, and
muscular capacity. They also overlap with other conditions that
are far more common, including depression and normal aging.
The added adipose tissue of hyperadrenocorticism is more
difficult to recognize in a person who is already obese. The
nervousness associated with hyperthyroidism is less apparent in
a thin, hyperkinetic man than in a person of moderate body
weight.
HISTORY
• As in most areas of medicine, precision of diagnosis and
economy of investigation begin with a carefully wrought history.
An open-ended question, combined with an attentive silence,
allows the patient to provide the background for the clinical
moment. After the patient has spoken spontaneously, the
physician provides a guided expansion of the information.
Details of timing, sequence, changes of diet or activity,
relationship to the menstrual cycle, changes in weight or size,
and alterations in mood or sleep patternall of these may provide
clues to underlying endocrine abnormality.
• Careful questioning about use of complementary and alternative
medicines
GENERAL EXAMINATION
• It is said that the history is 80% or more of clinical diagnosis,
and that is no less true in endocrine disorders than in general
medicine. Yet the physical examination is a critical element in
the process of arriving at a diagnosis, and here I want to call
particular attention to the first impression.
• The possibility of Cushing's syndrome, Addison's disease,
hyperthyroidism and hypothyroidism, acromegaly, polycystic
ovary syndrome, hypogonadism, and Turner's syndromethese
and other endocrine disorders should be considered from the
first moment one encounters a new patient.
TARGETED EXAMINATION
• The targeted physical examination of any consultant is an interesting
interplay of general and specific goals. Theoretically, any experienced
clinician should undertake a general examination and come to all the findings
pertinent to an underlying endocrine disorder. In fact, however, the physical
examination is greatly influenced by the hypotheses generated in the history.
• If a patient reports weight loss despite a good appetite, there is only a very
restricted differential diagnosis, principally malabsorption or
hypermetabolism. In doing a physical examination, therefore, I would pay
particular attention to signs of malabsorption (muscular wasting, vitamin
deficiencies, purpura) and to signs of thyroid disease with its generalized
hypermetabolism and localized autoimmune phenomena, including
ophthalmopathy.
DIRECT ASSESSMENT OF ENDOCRINE GLANDS
• Three endocrine glands are palpablethe thyroid, the testis, and the ovary.
Specific attention should be given to each of these.
• The thyroid gland should be approached first by inspection while the patient
swallowsfor size, symmetry, or localized enlargement. Many thyroid nodules
are visible, and inspection often calls attention to lesions that would be
missed on palpation. The thyroid should then be felt while the patient
swallows, from the front with your thumbs or from behind the patient with the
index and third fingers. It is crucial to keep your own fingers from moving
while the patient is swallowing. The principal observation is whether there is
diffuse enlargement of the thyroid gland
• Functioning tumors of the testis may be too small to be felt with the fingers,
and most internists and general physicians are not skilled in palpation of the
ovaries. For this reason, ultrasound and other forms of imaging have
become key features of gonadal evaluation and are discussed later.
• The size of one other endocrine gland, the pituitary, can be inferred from
physical examination for what Cushing called "neighborhood signs." As a
pituitary tumor or diffuse enlargement proceeds, it pushes up on the optic
chiasm from below, producing a bitemporal hemianopsia first manifested in
the upper quadrants, often to a blinking or flashing red light. This finding is
too subtle for the generalist's confidence, however, and pituitary assessment
depends on formal visual fields and imaging.
INDIRECT ASSESSMENT OF ENDOCRINE
STATUS
• Many consequences of hormone action can be detected on
physical examination; the results combine with the history to
produce a highly reliable differential diagnosis and thus an
informed basis for laboratory evaluation and imaging. Among
the things to be looked for are the eye signs and dermopathy of
Graves' disease, acanthosis nigricans as a clue to insulin
resistance, muscular wasting and tremor, changes in the voice
due to hypothyroidism or acromegaly, and a general impression
of nutrition and its adequacy or excess.
LABORATORY TESTING OF ENDOCRINE FUNCTION
• Modern endocrine laboratory evaluation began with the
introduction of radioimmunoassay by Berson and Yalow. The
precise measurement of hormone concentrations, determined by
competitive displacement of specific antibodies, was soon
succeeded by competitive binding assays and, more recently, by
immunofluorescent and radioluminescent determinations of even
greater sensitivity and specificity.
PULSATILE HORMONE SECRETION
• Many hormones are secreted in pulses rather than steadily. The
peaks or valleys of hormones secreted in pulsatile fashion, such
as luteinizing hormone or growth hormone, may fall above or
below the ostensibly normal range. If such a value is obtained
by chance, it can erroneously suggest hypofunction or
hyperfunction. Repeating the test with three samples drawn at
30-minute intervals and pooled can clarify this type of problem
DIURNAL VARIATION
• The hypothalamic-pituitary-adrenal axis of cortisol secretion is
typically maximal during the day and lower in the evening and
night. A plasma cortisol level of 12 μg/dL is normal at 8 AM, but
the same value at 8 PM reflects a loss of diurnal rhythm
resulting from either stress or hypercorticism. A plasma cortisol
sample drawn at midnight is an excellent test for evaluation of
overactive adrenal function.
CYCLIC VARIATION
• The menstrual cycle provides the most extreme "normal
variation" of any hormone level. From the first day of a
menstrual period, when estrogen levels may be
indistinguishable from those of a normal man, the level rises
extraordinarily rapidly and at the 14th day can be as high as in
early pregnancy. As a consequence, an estrogen level must be
evaluated in the light of the stage of the cycle at which it is
drawn.
AGE
• All clinicians are aware that gonadal hormones show marked
differences reflective of the individual's stage of life. It is not as
widely known that the adrenal hormone dehydroepiandrosterone
(DHEA) is barely secreted during childhood, is actively put out
by the adrenal glands from age 8 or so to age 55, and then
disappears as mysteriously as it came. At present, there is no
clear understanding of the physiologic role of its presence or
absence.
SLEEP ENTRAINMENT
• Both prolactin and growth hormone have a sleep-entrained
secretory pulse shortly after sleep begins. In people who work at
night and sleep during the day, this secretion is clearly related to
sleep and not to clock time.
HORMONE ANTAGONISM
• Certain hormones antagonize the effects of other hormones; it is
thus necessary to know the value of each hormone to interpret
the clinical phenomenon. The opposite effects of estrogen and
androgen on the male breast are a good example. A normal
testosterone level combined with an elevated estrogen level, or
a normal estrogen level but a decreased androgen level, easily
accounts for gynecomastia.
DYNAMIC TESTING
• Many endocrine glands have a basal secretory level and a
reserve secretion elicited by either a tropic hormone or a change
in metabolic or physiologic state. Cortisol secretion can increase
fivefold to 10-fold in response to stress or adrenocorticotropic
hormone (ACTH). Insulin release is stimulated by both glucose
and amino acids and by distinct pathways.
HORMONE AND METABOLITE INTERACTION
• Insulin is a good example of a hormone whose absolute level is
less meaningful than its relationship to the blood glucose level.
A plasma insulin of 70 is a normal response to a meal, when the
blood glucose level is rising. In contrast, an insulin value of 10 or
12 is abnormal (is not appropriately suppressed) if the glucose
level is 40 mg/dL. Indeed, the lower insulin level in a
hypoglycemic patient is distinct evidence of spontaneous
hyperinsulinism, such as in an islet cell tumor.
LABORATORY ERROR
• Laboratory error may seem too obvious a source of confusion to
mention, but it provides a reminder for an important caution
about laboratory testing. It is easy to be seduced by numbers
and to consider the laboratory report the final arbiter. In fact, it is
the history and physical examination, plus the clinician's
judgment, that establish the prior probability of a given
diagnosis.
IMAGING
• The extraordinary power of modern imaging, particularly ultrasound,
computed tomography (CT), and magnetic resonance imaging (MRI), has
enriched endocrinology as it has all of medicine.
• For one thing, several endocrine glands (the thyroid, the pituitary, and the
adrenals in particular) frequently contain clinically insignificant,
nonfunctioning adenomas and cysts.
• Second, functioning and nonfunctioning lesions other than in the thyroid
gland can be very difficult to distinguish from each other.
• In other words, one should be clear from hormone measurements, including
dynamic testing, whether the gland is overactive, underactive, or normal.
REFERENCES
• Handbook of diagnostic endocrinology edited by Janet E. Hall
and Lynnette K. Nieman (Contemporary endocrinology). 2011
• Williams textbook of endocrinology, Larsen, P.R. [et al.].10th ed.
2003
• Immunoendocrinology: Scientific and Clinical Aspects edited by
Eisenbarth G.S. 2011
You might also like
- Endocrine Equilibrium: Navigating the Hormonal Seas: Navigating the Whispers of Hormones: A Delicate Dance of BalanceFrom EverandEndocrine Equilibrium: Navigating the Hormonal Seas: Navigating the Whispers of Hormones: A Delicate Dance of BalanceNo ratings yet
- Endocrine System: A Tutorial Study GuideFrom EverandEndocrine System: A Tutorial Study GuideRating: 5 out of 5 stars5/5 (1)
- Introduction To Endocrinology: Dr. Jehad Al-ShuneigatDocument21 pagesIntroduction To Endocrinology: Dr. Jehad Al-Shuneigatyousef sarairehNo ratings yet
- Endocrinology 1st TrimesterDocument16 pagesEndocrinology 1st TrimesteriwennieNo ratings yet
- Study of The Endocrine SystemDocument225 pagesStudy of The Endocrine SystemHuzaifa RashidNo ratings yet
- Hormone: For Other Uses, SeeDocument4 pagesHormone: For Other Uses, SeePrasanna Nair ParameswaranNo ratings yet
- Hormones 859645Document34 pagesHormones 859645Musharaf RehmanNo ratings yet
- Introduction To HormonesDocument26 pagesIntroduction To HormonesHanif JundanaNo ratings yet
- Hormones:: Signaling MoleculesDocument20 pagesHormones:: Signaling MoleculesSangeeta DwivediNo ratings yet
- Introduction - CC3: Mechanism of Hormonal ActionDocument40 pagesIntroduction - CC3: Mechanism of Hormonal ActionAl-hadad AndromacheNo ratings yet
- EndocrinologyDocument35 pagesEndocrinologyNasif HussainNo ratings yet
- The study of hormones and homeostasisDocument102 pagesThe study of hormones and homeostasisJohn MkotaNo ratings yet
- Endocrine SystemDocument34 pagesEndocrine SystemNada DardeerNo ratings yet
- Biochemistry of HormonDocument16 pagesBiochemistry of HormonMuhamad SdeqNo ratings yet
- 439 en Intro 1Document74 pages439 en Intro 1Omotosho DavidNo ratings yet
- Endocrine SystemDocument41 pagesEndocrine SystemMerrin EncarnacionNo ratings yet
- Unit 1 Courselink NotesDocument27 pagesUnit 1 Courselink NotesLauren StamNo ratings yet
- The Endocrine System: A System of Chemical CommunicationDocument7 pagesThe Endocrine System: A System of Chemical CommunicationKaleem UllahNo ratings yet
- The Endocrine System: B. Pimentel, M.D. University of Makati College of NursingDocument54 pagesThe Endocrine System: B. Pimentel, M.D. University of Makati College of Nursingapi-19824701100% (1)
- Hormones, ClassificationDocument10 pagesHormones, ClassificationMenoNo ratings yet
- 1 - Hormonal Signal Transduction 2Document43 pages1 - Hormonal Signal Transduction 2Mohammed Mansour AbdullahNo ratings yet
- 1st Lec On Endo Physiology by DR RoomiDocument24 pages1st Lec On Endo Physiology by DR RoomiMudassar RoomiNo ratings yet
- Apbio 45 LectoutDocument15 pagesApbio 45 LectoutSimone Alexis GuinocorNo ratings yet
- 5endocrlect3 191011165740Document55 pages5endocrlect3 191011165740OROKE JOHN EJENo ratings yet
- Introduction To EndocrinologyDocument27 pagesIntroduction To Endocrinologyjimmybarack27No ratings yet
- Endocrine System OverviewDocument26 pagesEndocrine System OverviewJuwitaNo ratings yet
- Endrocrinology - PPTX Version 1Document40 pagesEndrocrinology - PPTX Version 1Abishek BhadraNo ratings yet
- Anatomy and Physiology,: Lecture OutlineDocument79 pagesAnatomy and Physiology,: Lecture OutlineJorelyn Giron NaniongNo ratings yet
- Endocrine System OverviewDocument11 pagesEndocrine System OverviewwasimsafdarNo ratings yet
- Lewis: Medical-Surgical Nursing, 10 Edition: Assessment of Endocrine System Key PointsDocument3 pagesLewis: Medical-Surgical Nursing, 10 Edition: Assessment of Endocrine System Key PointsDeo FactuarNo ratings yet
- ENDOCRINOLOGY in Orthodontics - Dr. HibaDocument86 pagesENDOCRINOLOGY in Orthodontics - Dr. HibaHiba AbdullahNo ratings yet
- Introduction Endocrine Physiology 2Document52 pagesIntroduction Endocrine Physiology 2IshaqNo ratings yet
- Endocrine Physiology - Part 1Document40 pagesEndocrine Physiology - Part 1Terrence Beniasi CharumbiraNo ratings yet
- E N D o C R I N o L o G Y: Dr. Victoria G. Giango Chair, Dept. of PhysiologyDocument23 pagesE N D o C R I N o L o G Y: Dr. Victoria G. Giango Chair, Dept. of Physiologyanon-271548No ratings yet
- Endocrine Glands and HormonesDocument46 pagesEndocrine Glands and Hormonesfisherp1No ratings yet
- Care of Clients With Problem On EndocrineDocument48 pagesCare of Clients With Problem On EndocrineAngel CauilanNo ratings yet
- Endocrine System PDFDocument38 pagesEndocrine System PDFUlfat NiazyNo ratings yet
- Endocrine System GuideDocument37 pagesEndocrine System GuideKochaMsangiNo ratings yet
- Endo Lect - OverviewDocument24 pagesEndo Lect - OverviewdoctorrfarrukhNo ratings yet
- Hormone: For Other Uses, SeeDocument5 pagesHormone: For Other Uses, SeeEki Ryan SetiowatiNo ratings yet
- General Principles of Metabolism Regulation. HormonesDocument90 pagesGeneral Principles of Metabolism Regulation. HormonesМохіт Кумар ЯмпатіNo ratings yet
- 1 - Endocrine 1 (Introduction) - MedicineDocument36 pages1 - Endocrine 1 (Introduction) - MedicineBHUWAN BASKOTANo ratings yet
- Introduction To Endocrine SystemDocument91 pagesIntroduction To Endocrine SystemGifti DemisseNo ratings yet
- Understanding Endocrine Disorders and Their Diagnosis & ManagementDocument252 pagesUnderstanding Endocrine Disorders and Their Diagnosis & Managementyuuki konnoNo ratings yet
- Endocrine System - MorphophysiologyDocument88 pagesEndocrine System - MorphophysiologyKarina Bustillo100% (1)
- Chemistry ProjectDocument23 pagesChemistry Projecttwinklelee123450% (2)
- Chapter 48: Nursing Assessment: Endocrine System: Structures and FunctionsDocument3 pagesChapter 48: Nursing Assessment: Endocrine System: Structures and FunctionsjefrocNo ratings yet
- Principles of endocrinology scope and functionsDocument25 pagesPrinciples of endocrinology scope and functionsRyan James Lorenzo MiguelNo ratings yet
- Prof. N. Syabbalo: MB., CHB., PHD., FCCP., FRS., Fiba Professor of Physiology & MedicineDocument45 pagesProf. N. Syabbalo: MB., CHB., PHD., FCCP., FRS., Fiba Professor of Physiology & MedicineHomeground entertainmentNo ratings yet
- Basic Concempts of Endocrine RegulationDocument50 pagesBasic Concempts of Endocrine RegulationHomeground entertainmentNo ratings yet
- Endocrine Physiology OverviewDocument166 pagesEndocrine Physiology OverviewdrpnnreddyNo ratings yet
- Endocrine System: Dr. Annette M. Parrott GPC BIOL1612Document34 pagesEndocrine System: Dr. Annette M. Parrott GPC BIOL1612Nathan TaylorNo ratings yet
- EndocrinologyDocument74 pagesEndocrinologysyedahaleemajunaidsNo ratings yet
- The Endocrine System: HormonesDocument948 pagesThe Endocrine System: HormonesMahaaaNo ratings yet
- Hormonal Regulation of ExerciseDocument11 pagesHormonal Regulation of ExerciseMozil Fadzil KamarudinNo ratings yet
- Nervous Vs HormoneDocument106 pagesNervous Vs HormoneakupunadaNo ratings yet
- PD22 Hap1 L03Document33 pagesPD22 Hap1 L03Ka Yan LAUNo ratings yet
- Learning JournalDocument82 pagesLearning JournalAnnapurna RavelNo ratings yet
- Reproductive SystemDocument114 pagesReproductive SystembookaccountNo ratings yet
- HORMONESDocument65 pagesHORMONESsoumya palavalasaNo ratings yet
- Pertemuan 13 - Sirosis HepatisDocument18 pagesPertemuan 13 - Sirosis HepatisYudiatmaNo ratings yet
- CASP Clinical Prediction Rule Checklist DownloadDocument5 pagesCASP Clinical Prediction Rule Checklist DownloadbintangNo ratings yet
- Kidneystones 02012012 PDFDocument7 pagesKidneystones 02012012 PDFYudiatmaNo ratings yet
- Sesi2 Systematic Review Dengan PrismaDocument28 pagesSesi2 Systematic Review Dengan PrismaBalqis BaslemanNo ratings yet
- Update On Subclinical Hyperthyroidism 15-4-2011 AAFP p933Document6 pagesUpdate On Subclinical Hyperthyroidism 15-4-2011 AAFP p933micheal1960No ratings yet
- The Effect of Resistance Training On Thyroid HormonesDocument5 pagesThe Effect of Resistance Training On Thyroid Hormonesroyal_rahulNo ratings yet
- Medical Terminologies and AbbreviationDocument4 pagesMedical Terminologies and AbbreviationHana LunariaNo ratings yet
- David - Perlmutter-The Thyroid ConnectionDocument22 pagesDavid - Perlmutter-The Thyroid Connectionfumi100% (1)
- Case Studies On Major Concepts: MetabolismDocument37 pagesCase Studies On Major Concepts: MetabolismJek Dela CruzNo ratings yet
- Ancient Healing Techniques A Course in - Douglas DDocument157 pagesAncient Healing Techniques A Course in - Douglas DAni Danna93% (28)
- Congenital HypothyroidismDocument22 pagesCongenital HypothyroidismFredy Wirya AtmajaNo ratings yet
- Chemical Coordination and IntegrationDocument17 pagesChemical Coordination and IntegrationHemant KumarNo ratings yet
- Peds HESI Dec 2018 PDFDocument11 pagesPeds HESI Dec 2018 PDFMariya ZafraniNo ratings yet
- Endocrine System NotesDocument6 pagesEndocrine System NotesHannah Grace CorveraNo ratings yet
- Drug Study - LevothyroxineDocument1 pageDrug Study - LevothyroxineCarla Tongson Maravilla100% (1)
- PHARMACOLOGYDocument36 pagesPHARMACOLOGYjanr123456100% (1)
- 07 EndocrineDocument44 pages07 Endocrineandirio7486No ratings yet
- High-Yield Endocrine OverviewDocument33 pagesHigh-Yield Endocrine OverviewVictoria MorenoNo ratings yet
- Sharma 2017Document6 pagesSharma 2017asfwegereNo ratings yet
- Mrs Parveen Inamdar 29 02 2020 06 16 15 AM PDFDocument3 pagesMrs Parveen Inamdar 29 02 2020 06 16 15 AM PDFmalhari katakdoundNo ratings yet
- Chemical Classification of Hormones: Steroids, Peptides, Eicosanoids & MoreDocument9 pagesChemical Classification of Hormones: Steroids, Peptides, Eicosanoids & Morevishal sharmaNo ratings yet
- Biophysics of Earthing Grounding The Human Body 2015Document26 pagesBiophysics of Earthing Grounding The Human Body 2015Mayna100% (1)
- Nootropics Expert Secrets of The Optimized Brain 3rd Editon PDFDocument84 pagesNootropics Expert Secrets of The Optimized Brain 3rd Editon PDFAleksandar Novakovic100% (5)
- Package Insert - 9300800 - E - en - 30459 FT4 PDFDocument6 pagesPackage Insert - 9300800 - E - en - 30459 FT4 PDFadybaila4680No ratings yet
- Congenital HypothyroidismDocument21 pagesCongenital HypothyroidismDanna GarcíaNo ratings yet
- IM Essentials QuestionsDocument12 pagesIM Essentials QuestionsAakash Shah100% (1)
- Mag Nascent IodineDocument3 pagesMag Nascent IodineNavigator4life100% (1)
- GoljanDocument59 pagesGoljanShri ReddyNo ratings yet
- Monobind CatalogDocument37 pagesMonobind CatalogRobert Caballero Bardales100% (2)
- Noureen Zawar Family Medicine FMH Oct 21, 2017Document32 pagesNoureen Zawar Family Medicine FMH Oct 21, 2017Noureen ZawarNo ratings yet
- Tiroiditis Subakut PDFDocument8 pagesTiroiditis Subakut PDFAdeh MahardikaNo ratings yet
- All Medical Questions and Answers - FmyDocument30 pagesAll Medical Questions and Answers - FmyOloya Vincent100% (1)
- 2022 ESHRE RPL Guideline Update 2022 Draft For Stakeholder ReviewDocument157 pages2022 ESHRE RPL Guideline Update 2022 Draft For Stakeholder ReviewDairo PintoNo ratings yet