Professional Documents
Culture Documents
Basic Concempts of Endocrine Regulation
Uploaded by
Homeground entertainment0 ratings0% found this document useful (0 votes)
10 views50 pagesOriginal Title
BASIC CONCEMPTS OF ENDOCRINE REGULATION
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
10 views50 pagesBasic Concempts of Endocrine Regulation
Uploaded by
Homeground entertainmentCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 50
Prof. N.
SYABBALO
MB., ChB., PhD., FCCP., FRS., FIBA
Professor of Physiology & Medicine
BASIC CONCEPTS OF ENDOCRINE
REGULATION
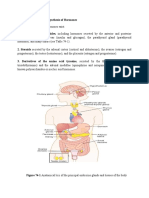
The unique feature of endocrine physiology is that,
unlike other physiological systems, the endocrine
system cannot be defined along anatomic lines
Rather, the endocrine system is a distributed system of
glands and circulating messengers (hormones) that is
often simulated by the central nervous system and/or
autonomic nervous system
Hormones comprise steroids, amines, and peptides
Peptides are by far the most numerous
BASIC CONCEPTS OF ENDOCRINE
REGULATION
They include hormones secreted by the anterior and
posterior pituitary gland, the pancreas (insulin and
glucagon), the parathyroid gland (parathyroid hormone)
and many others
Steroids are secreted by the adrenal cortex (cortisol and
aldosterone), the ovaries (estrogen and progesterone), the
testes (testosterone), and the placenta (estrogen and
progesterone)
Derivatives of the amino acid tyrosine are secreted by the
thyroid gland (thyroxine and triodothyronine), and the
adrenal medulla (epinephrine and norepinephrine)
EVOLUTION OF HORMONES AND
THEIR ACTIONS ON TARGET CELLS
Many hormones can be grouped into families
reflecting their structural similarities as well as the
similarities of the receptors they activate
However, the number of hormones and their diversity
increase as one moves from simple to higher life forms
This reflects the added challenges in providing for
homeostasis in more complex organisms
For example, among the peptides hormones, several
are heterodimers that share a common σ chain, with
specificity being conferred by the chain
EVOLUTION OF HORMONES AND
THEIR ACTIONS ON TARGET CELLS
In the specific cases of thyroid-stimulating hormone
(TSH), follicular-stimulating hormone (FSH), and
luteinizing hormone (LH), there is evidence that the
distinct chains arose from a series of duplications of a
common ancestral gene
For these and other hormones, moreover, this
molecular evolution implies that hormone receptors
also needed to evolve to allow for spreading of
hormone actions/specificity
EVOLUTION OF HORMONES AND
THEIR ACTIONS ON TARGET CELLS
This was accomplished by co-evolution of the basic G-
protein coupled receptors (GPCR) and receptor
tyrosine kinases that mediate the effects of peptide and
amine hormones that act on the cell surface
The underling ancestral relationship sometimes re-
emerge, however, in the cross-reactivity that may be seen
when hormones rise to unusually high levels (eg,
endocrine tumours)
Steroids and thyroid hormones are distinguished by their
predominantly intracellular sites of actions, since they
can diffuse freely through the cell membranes
EVOLUTION OF HORMONES AND
THEIR ACTIONS ON TARGET CELLS
They bind to a largely cytoplasmic proteins known as
nuclear receptors
Upon ligand binding, the receptor-ligand complex
translocates to the nucleus where it either
homodimerizes, or associates with a distinct liganded
nuclear receptor to form a heterodimer
In either case, the dimer bind to DNA to either increase
or decrease gene transcription in the target tissue
Individual members of the nuclear receptor family have
a considerable degree of homology
EVOLUTION OF HORMONES AND
THEIR ACTIONS ON TARGET CELLS
This, perhaps implying a common ancestral gene, and
share many functional domains, such as the zinc
fingers that permit DNA binding
However, sequence variations allow for ligand
specificity as well as binding to specific DNA motifs
In this way, the transcription of distinct genes is
regulated by individual hormones
HORMONE SECRETION
SYNTHESIS AND PROCESSING
The regulation of hormone synthesis, depends on
their chemical nature
Polypeptides and protein hormones
The synthesis of all of the protein/peptide hormones
as well as hormone receptors, is subject to a normal
mechanisms of transcriptional control in the cell
In addition, there is provision for exquisitely specific
regulation by other hormones, since the regulatory
regions of many peptide hormone genes contain
binding motifs for nuclear receptors
HORMONE SECRETION
SYNTHESIS AND PROCESSING
Peptide are synthesized on the rough end of the
endoplasmic reticulum of different endocrine cells in
the same fashion as most other proteins
They are usually synthesized at first as larger proteins
that are not biologically active (preprohormones)
and are cleaved to form smaller prohormones in the
endoplasmic reticulum by specific proteases
In some cases, multiple hormones may be derived
from the same initial precursor, depending on the
specific processing steps present in a given cell
HORMONE SECRETION
SYNTHESIS AND PROCESSING
Presumably this provides for a form of genetic
‘economy’
Prohormones are then transferred to the Golgi
apparatus for packaging into secretory vesicles
In this process, enzymes in the vesicles leave the
prohormones to produce smaller, biologically active
hormones and inactive fragments
The vesicles are stored within the cytoplasm, and
many are bound to the cell membranes until their
secretion is needed
HORMONE SECRETION
SYNTHESIS AND PROCESSING
Secretion of the hormone occurs when the secretory
vesicles fuse with the cell membrane and the granular
contents are extruded into the interstitial fluid or directly
into blood stream by exocytosis
The stimulus for exocytosis is an increase in cystolic
calcium concentration caused by depolarization of the
plasma membrane
In other instances, stimulation of endocrine cell surface
receptor causes increased cyclic adenosine
monophosphate (cAMP) and subsequently activation of
protein kinase that initiate secretion of the hormone
HORMONE SECRETION
SYNTHESIS AND PROCESSING
The peptide hormones are water soluble, allowing
them to enter the circulation system easily, where they
are carried to their target tissues
Steroid hormones
For steroid hormones, synthesis is controlled
indirectly by regulating the production of key
synthetic enzymes, as well as by substrate availability
The chemical structure of steroid hormones is similar
to that of cholesterol, and in most instances they are
synthesized from cholesterol itself
HORMONE SECRETION
SYNTHESIS AND PROCESSING
They are lipid soluble and consist of three cyclohexyl
rings and one cyclopentyl ring combined into a single
structure
Although there is usually very little hormone storage
in steroid-producing endocrine cells, large amount of
cholesterol ester in cytoplasm vacuoles can be rapidly
mobilized for steroid synthesis after a stimulus
Most of the cholesterol in steroid-producing cells
comes from the plasma, but there is also de novo
synthesis of cholesterol in steroid-producing cells
HORMONE SECRETION
SYNTHESIS AND PROCESSING
Because the steroids are highly lipid soluble, once they
are synthesized, they simply diffuse across the cell
membrane and enter the interstitial fluid and then the
blood
Amine hormones
The two groups of hormones derived from tyrosine,
the thyroid and the adrenal medullary hormones are
formed by the actions of enzymes in the cytoplasmic
compartments of the glandular cells
HORMONE SECRETION
SYNTHESIS AND PROCESSING
The thyroid hormones are synthesized and stored in the
thyroid gland and incorporated into macromolecules of
the protein thyroglobulin which is stored in large
follicles within the thyroid gland
Hormone secretion occurs when the amines are split
from thyroglobulin, and the free hormones (T 3 and T4)
are released into the blood stream
After entering the blood, most of the thyroid hormones
combine with plasma proteins, especially thyroxine-
biding globulin, which slowly release the hormones to
the target tissues
HORMONE SECRETION
SYNTHESIS AND PROCESSING
Epinephrine and norepinephrine are formed in the
adrenal medulla, which secretes about four times more
epinephrine than norepinephrine
Catecholamines are stored in preformed vesicles and
stored until secreted
Similar to the protein hormones, catecholamines are
also released from adrenal medullary cells by exocytosis
Once they enter the circulation, they can exist in plasma
in free form or in conjugation with other substances
HORMONE SECRETION
SYNTHESIS AND PROCESSING
Most importantly is that, hormone precursors
themselves are typically inactive
This may be a mechanism that provides for an
additional measure of regulatory control, or, in the
case of thyroid hormones, may dictate the site of
highest hormone availability
In addition, there is provision for exquisitely specific
regulation by other hormones, since the regulatory
regions of many peptide hormone genes contain
binding motifs for nuclear receptors
HORMONE SECRETION
SYNTHESIS AND PROCESSING
For example, thyroid hormone directly suppresses
TSH expression via the thyroid hormone receptor
These specific mechanisms to regulate hormone
transcription are essential to the function of feedback
loops
In some cases, the abundance of selected hormones
may also be regulated via effects on translation
For example, elevated levels of circulating glucose
stimulate the translation of insulin mRNA
HORMONE SECRETION
SYNTHESIS AND PROCESSING
These effects are mediated by the ability of glucose to
increase the interaction of insulin mRNA with specific
RNA binding proteins, which increase its stability and
enhances its translation
The net effect is a more precise and timely regulation
of insulin levels, and thus energy metabolism, than
could be accomplished with transcriptional regulation
alone
HORMONE SECRETION
SYNTHESIS AND PROCESSING
The precursor for peptide hormones are processed
through the cellular machinery that handles proteins
destined for export, including trafficking through special
vesicles where the propeptide form can be cleaved to the
active hormones
Mature hormones are also subjected to a variety of
posttranslational processing steps, such as glycosylation,
which can influence their ultimate biological activity
and/or stability in the circulation
Ultimately, all hormones enter either the constitutive or
regulated secretory pathway
SECRETION
The secretion of many hormones is via a process of
exocytosis of stored granules
The exocytotic machinery is activated when the cell
type that synthesizes and stores the hormone in
question is activated by a specific signal, such as a
neurotransmitter or peptide releasing factor
One should, however, contrast the secretion of stored
hormones with that of those that are continuously
released by diffusion (eg, steroids)
SECRETION
Control of secretion of the later molecule occurs via
kinetic influence on synthetic enzymes or carrier
proteins involved in the hormone production
For example, the steroidogenic acute regulatory
protein (StAR) is a labile protein whose expression,
activation, and deactivation are regulated by
intracellular signaling cascades and their effectors,
including a variety of protein kinases and phosphates
StAR traffics cholesterol form the outer to the inner
membrane leaflet of the mitochondrion
SECRETION
Because this is a rate-limiting first step in the
synthesis of the steroid precursor, pregnenolone, this
arrangement permits changes in the rate of steroid
synthesis, and thus secretion, in response to
homeostatic cues such as trophic hormones, cytokines
and stress (Figure 16-1)
An additional complexity related to hormone
secretion relates to the fact that some hormones are
secreted in a pulsatile fashion
SECRETION
Pulsatile secretion is the normal pattern for the
gonadotrophins, LH and FSH, with major pulses
released every 1-2 hours depending on the phase of the
menstrual cycle
Growth hormone is also secreted in pulsatile fashion,
with undetectable levels between pulses
Secretion rates may peak and ebb relative to circadian
rhythms, in response to the timing of meals, or as
regulated by other pattern generators whose
periodicity may range from milliseconds to years
SECRETION
Circadian means changes over 24 hours of the day-
night cycle and is best shown by the pituitary-adrenal
axis
For example, cortisol levels are highest in the morning
and lowest in overnight
Additionally, cortisol release is pulsatile, following the
pulsatility of pituitary ACTH
The menstrual cycle is an example of a longer and
more complex (28-day biological rhythm)
SECRETION
Pulsatile secretion is often related to the activity of
oscillators in the hypothalamus that regulate the
membrane potential of neurons
Which in turn secret bursts of hormone releasing factors
into the hypophysial blood flow that then cause the
release of pituitary and other downstream hormones in a
pulsatile fashion
There is evidence that these hormones pulses convey
different information to the target tissues that they act
upon than steady exposure to a single concentration of
the hormone
SECRETION
Therapeutically, pulsatile secretion may pose
challenges if, due to deficiency, it proves necessary to
replace a pulsatile hormone that is normally secreted
in this way
Continuous secretion is typical of thyroid
hormones, with a half-life of 7-1o days for T4 and 6-10
hours for T3, and with little variation over the day,
month and year
FIGURE 16-1
Regulation of steroid biosynthesis by StAR
Extracellular signals activate intracellular kinases that,
in turn, phosphorylate transcription factors that
upregulate StAR expression
StAR is activated by phosphorylation, and facilitates
transfer of cholesterol from the outer to inner
mitochondrial membrane leaflet
This then allows entry of cholesterol into the steroid
biosynthetic pathway, beginning with pregnenolone
HORMONE TRANSPORT IN BLOOD
In addition to the rate of secretion and its nature (steady
vs pulsatile), a number of factors influence the circulating
levels of hormones
These include the rates of hormone degradation and/or
uptake
Receptor binding and availability of receptors, and the
affinity of a given hormone for plasma carriers
Stability influences the circulating half-life of a given
hormone and has therapeutic implications for hormone
replacement therapy, in addition to those posed by
pulsatile secretion
HORMONE TRANSPORT IN BLOOD
Plasma carriers for specific hormones have a number
of important physiological function
First, they serve as a reservoir of inactive hormone and
thus provide a hormonal reserve
Bound hormones are typically prevented from
degradation or uptake
Ultimately, plasma carriers may be vital in modulating
levels of the free hormones
HORMONE TRANSPORT IN BLOOD
Typically, it is only the free hormone that is
biologically active in target tissues or can mediate
feedback regulation since it is the only form able to
access the extravascular compartment
Thus, the bound hormone reservoir can allow
fluctuation in hormonal levels to be smoothed over
time
Plasma carriers also restrict the access of the hormone
to some sites
HORMONE TRANSPORT IN BLOOD
Catecholamines and most peptide hormones are
soluble in plasma and are transported as such
Norepinephrine and epinephrine, are secreted within
seconds after the gland is stimulated, and they may
develop full action within another few seconds to
minutes
In contrast steroid hormones are hydrophobic and are
mostly bound to large proteins called steroid binding
proteins (SBP), which are synthesized in the liver
HORMONE TRANSPORT IN BLOOD
As a result, only small amounts of the free hormone
are dissolved in plasma
Specifically, sex hormone-binding globulin (SHBG)
is a glycoprotein that binds to the sex hormones,
testosterone and 17β-estradiol
Progesterone, cortisol, and other corticosteroids are
bound by transcortin
The SBP hormone complex and the free hormone are
in equilibrium in the plasma, and only the free
hormone is able to diffuse across cell membranes
HORMONE TRANSPORT IN BLOOD
SBP have three main functions: they increase the
solubility of lipid based hormone in the blood
They reduce the rate of hormone loss in the urine by
preventing the hormone from being filtered in the
kidney
And they provide a source of hormone in the
bloodstream that can release free hormone as the
equilibrium change
HORMONE TRANSPORT IN BLOOD
An additional way to regulate the availability of hormones
that bind to a carrier proteins, such as steroids, is to
regulate the expression and secretion of the carrier
proteins themselves
This is a critical mechanism that regulates the
bioavailability of thyroid hormones, for example
Thyroid hormones are transported in the circulation by a
carrier protein thyroxine-binding globulin
In a pathophysiological setting, some medications can
alter the levels of binding proteins or displace hormones
that are bound to them
HORMONE TRANSPORT IN BLOOD
This is a critical mechanism that regulates the
bioavailability of thyroid hormones, for example
In addition, some binding proteins are promiscuous and
bind multiple hormones (eg, SHBG)
These observations may have clinical implications for
endocrine homeostasis, since free hormones are needed
to feedback and control their rates of synthesis and
secretion
Finally, the anatomic relationship of sites of release and
action of hormones may play a key role in their regulation
HORMONE TRANSPORT IN BLOOD
For example, a number of hormones are destroyed by
passage through the pulmonary circulation or the liver
This may markedly curtail the temporal window within
which a given hormone can act
Concentration of hormones in the circulation
The concentration of hormones required to control most
metabolic and endocrine functions are incredibly small
Their concentration in the blood range from as little as 1
picogram in each millilitre of blood up to at most a few
milligrams per millimetre of blood
HORMONE TRANSPORT IN BLOOD
Similarly, the rates of secretion of various hormones
are extremely small, measured in micrograms or
milligrams per day
The rate of action of hormones also varies
Norepinephrine and epinephrine may develop full
action within seconds to minutes after being secreted,
the actions of other hormones, such as thyroxine or
growth hormones, may require months for full effect
CLINICAL BOX 16-1
Breast cancer
Breast cancer is the most common malignancy of
women, with about 1 million new cases diagnosed each
year world-wide
The proliferation of more than two-thirds of breast
tumours are driven by the ovarian hormone, oestrogen,
and about one-third of patients, the breast cancer will
express receptors for oestrogen and progesterone
This is by virtue of the fact that the tumour cells express
high levels of posttranslationally modified oestrogen
receptors (ER)
CLINICAL BOX 16-1
Breast cancer
The clinical significance of these molecular findings
has been known for more than 100 years, since the
Scottish surgeon, Sir Thomas Beatson, reported
delayed disease progression in patients with advanced
breast cancer following removal of their ovaries in
premenopausal women
The extent of exposure to ovulatory cycles is one of
most important endogenous causes associated with a
high risk for development of sporadic breast cancer
CLINICAL BOX 16-1
Breast cancer
However, although the association of oestrogen in the
development of breast cancer is well established, the
fundamental mechanism(s) by which this hormone
modulate cell growth and tumour development are not
yet clear
It is known from in vitro and in vivo studies that the
mechanism of oestrogen action is mediated through
the binding to the ER
Oestrogen receptors in turn binds specific enhancer
regions on the DNA and regulates gene transcription
CLINICAL BOX 16-1
Breast cancer
The interaction of oestrogen and its receptors and the
recruitment of accessory cofactor proteins has been
the focus of intense recent research
In modern times, determination of whether a given
breast cancer is, or is not, ER-positive is a critical
diagnostic test that guides treatment decisions, as well
as an important prognosticator
ER-positive tumours are typically of lower grade, and
patients with such tumours have improved survival
CLINICAL BOX 16-1
Breast cancer
The latter is likely due, at least in part, to the
availability of excellent treatment options for ER-
positive tumours compared with those that are ER-
negative
On the other hand, the oestrogen receptor negative,
progesterone receptor (R) negative, mutated EGFR
(Her2) negative, triple negative phenotype (basal type)
is associated with a poor prognosis than the luminal
types A and B
THERAPEUTIC HIGHLIGHTS
Oestrogen-responsive breast tumours are dependent
on the presence of the hormone for growth
In modern times, cells can be deprived of the effects of
oestrogen pharmacologically, rather than resorting to
oophorectomy
Premenopausal women
In premenopausal women, a reduction in oestrogens
can be achieved via pituitary downregulation using
gonadotropin-releasing hormone (GnRH) analogues
such as goserelin or leuprorelin
THERAPEUTIC HIGHLIGHTS
Tamoxifen a mixed agonist and antagonist of oestrogen
action on the ER, and related agents that specifically
inhibit the oestrogen receptor may hasten its degradation
The more recent drug fulvestrant is a more selective
oestrogen modulator (SERM) and is also used for the
treatment of breast cancer
Synthetic progestogens, eg, medroxyrogesterone acetate
and megestrol, have a direct effect on breast tumour cells
through progesterone receptors
They can be as effective as tamoxifen in metastatic breast
cancer
THERAPEUTIC HIGHLIGHTS
Postmenopausal women
In postmenopausal women, where oestrogen is
derived from the metabolism of testosterone to
estrone in extragonadal tissues (ie, subcutaneous fat)
rather than from the ovaries, aromatase inhibitors
inhibits the conversion of androgens to oestrogen
The aromatase inhibitors, anastrozole, letrozole
and exemestane, reduce circulating oestrogen levels
and synthesis in tumour cells
THERAPEUTIC HIGHLIGHTS
They have shown greater efficacy than tamoxifen in the
treatment of metastatic breast cancer and equivalent in
adjuvant setting
In general, treatment of breast cancer includes surgery:
local excission, segmental mastectomy or simple
mastectomy with or without reconstruction, radiation
therapy, chemotherapy and hormone-modulating
therapy, e.g., taxomifen
Patients with established metastatic disease may require
endocrine therapy, chemotherapy and radiotherapy
THERAPEUTIC HIGHLIGHTS
The anti-oestrogen drug taxomifen is effective when
used as a single agent or when used in combination
with other chemotherapeutic agents in patients who
are postmenopausal
Chemotherapy is used for patients who lack features
of hormone responsive disease or who fail to respond
to endocrine therapy or who require rapid response if
at risk of, eg, liver or respiratory failure
There is no advantage in combining more than two
drugs at a time
THERAPEUTIC HIGHLIGHTS
There is very little difference in efficacy between the different
regimens for metastatic disease, with response rates varying
from 40% to 60% for median duration of 8-10 months
The most common regimens with either single agent or
doublet combination include:
MM: mitoxantrone and methotraxate
AC/EC: doxorubicin or epirubicin and cyclophosphamide
DC: docetaxel and gemcitabine
PG: paclitaxel and gemcitabine
Vinorelbine, carbolatin, mitomycin and eribulin
You might also like
- Prof. N. Syabbalo: MB., CHB., PHD., FCCP., FRS., Fiba Professor of Physiology & MedicineDocument45 pagesProf. N. Syabbalo: MB., CHB., PHD., FCCP., FRS., Fiba Professor of Physiology & MedicineHomeground entertainmentNo ratings yet
- Hormone: For Other Uses, SeeDocument5 pagesHormone: For Other Uses, SeeEki Ryan SetiowatiNo ratings yet
- Adrenocorticotropic Hormone (ACTH) From The Anterior Pituitary Gland Specifically StimulatesDocument2 pagesAdrenocorticotropic Hormone (ACTH) From The Anterior Pituitary Gland Specifically StimulatesStjepan ErženNo ratings yet
- Unit 1 Courselink NotesDocument27 pagesUnit 1 Courselink NotesLauren StamNo ratings yet
- Hormonal ActionDocument37 pagesHormonal ActionHomeground entertainmentNo ratings yet
- Study of The Endocrine SystemDocument225 pagesStudy of The Endocrine SystemHuzaifa RashidNo ratings yet
- Endrocrinology - PPTX Version 1Document40 pagesEndrocrinology - PPTX Version 1Abishek BhadraNo ratings yet
- Hormone: For Other Uses, SeeDocument4 pagesHormone: For Other Uses, SeePrasanna Nair ParameswaranNo ratings yet
- What Are HormonesDocument16 pagesWhat Are HormonesHarry RoyNo ratings yet
- The Endocrine System: A System of Chemical CommunicationDocument7 pagesThe Endocrine System: A System of Chemical CommunicationKaleem UllahNo ratings yet
- Biochemistry of HormonDocument16 pagesBiochemistry of HormonMuhamad SdeqNo ratings yet
- Hormones, ClassificationDocument10 pagesHormones, ClassificationMenoNo ratings yet
- EndocrinologyDocument35 pagesEndocrinologyNasif HussainNo ratings yet
- 1 - Introduction EndocrinologyDocument40 pages1 - Introduction EndocrinologyBHUWAN BASKOTANo ratings yet
- The Endocrine System: Hormones, Receptors and RegulationDocument47 pagesThe Endocrine System: Hormones, Receptors and RegulationRifi OktasiaNo ratings yet
- Chemistry ProjectDocument23 pagesChemistry Projecttwinklelee123450% (2)
- Chemical Structure and Synthesis of HormonesDocument6 pagesChemical Structure and Synthesis of HormonesFAHEEM UD DINNo ratings yet
- Endocrine System AssesmentDocument51 pagesEndocrine System AssesmentYudiatma100% (1)
- Endocrine Physiology Lecture 2Document9 pagesEndocrine Physiology Lecture 2Tofunmi Kunle-KunbiNo ratings yet
- Introduction To Endocrinology: Dr. Jehad Al-ShuneigatDocument21 pagesIntroduction To Endocrinology: Dr. Jehad Al-Shuneigatyousef sarairehNo ratings yet
- 4 Endocrine 1Document18 pages4 Endocrine 1ShenNo ratings yet
- Endocrine Anatomy and PhysiologyDocument10 pagesEndocrine Anatomy and PhysiologyJojo JustoNo ratings yet
- Biology ProjectDocument27 pagesBiology Projectravifact0% (2)
- SGD Physiology Endocrine and MetabolismDocument7 pagesSGD Physiology Endocrine and MetabolismTinesh RajahNo ratings yet
- ABS Endocrinology PDFDocument59 pagesABS Endocrinology PDFMichael Olivier WNo ratings yet
- EndocrinologyDocument223 pagesEndocrinologyMr mk RollinsNo ratings yet
- SUMAN - TAMANG2021-02-14Regulation of Hormone ActionDocument43 pagesSUMAN - TAMANG2021-02-14Regulation of Hormone Actions vNo ratings yet
- Coordination of Body Functions by Chemical MessengerDocument6 pagesCoordination of Body Functions by Chemical MessengerHorain FatimaNo ratings yet
- Introduction - CC3: Mechanism of Hormonal ActionDocument40 pagesIntroduction - CC3: Mechanism of Hormonal ActionAl-hadad AndromacheNo ratings yet
- Endocrinology 1st TrimesterDocument16 pagesEndocrinology 1st TrimesteriwennieNo ratings yet
- Hormonal Management of Male InfertilityDocument22 pagesHormonal Management of Male Infertilitydranibalurologoencancun.mxNo ratings yet
- Chapter - 17 PhysiologyDocument49 pagesChapter - 17 PhysiologyAhsanullah PathanNo ratings yet
- Endocrine SystemDocument34 pagesEndocrine SystemNada DardeerNo ratings yet
- Hormones:: Signaling MoleculesDocument20 pagesHormones:: Signaling MoleculesSangeeta DwivediNo ratings yet
- Endocrine SystemDocument41 pagesEndocrine SystemMerrin EncarnacionNo ratings yet
- Endocrine System OutlineDocument17 pagesEndocrine System OutlineEsthermae PanadenNo ratings yet
- E N D o C R I N o L o G Y: Dr. Victoria G. Giango Chair, Dept. of PhysiologyDocument23 pagesE N D o C R I N o L o G Y: Dr. Victoria G. Giango Chair, Dept. of Physiologyanon-271548No ratings yet
- Lec 8 - Hormones PDFDocument13 pagesLec 8 - Hormones PDFrajeshNo ratings yet
- Hormones EssayDocument2 pagesHormones Essayapi-254545870No ratings yet
- Learning JournalDocument82 pagesLearning JournalAnnapurna RavelNo ratings yet
- Anatomy and Physiology-Endocrine SystemDocument5 pagesAnatomy and Physiology-Endocrine SystemEixid Enna YeLikNo ratings yet
- Overview of Endocrine SystemDocument16 pagesOverview of Endocrine System2023ph17No ratings yet
- HORMONESDocument9 pagesHORMONESSARA M SNo ratings yet
- UntitledDocument12 pagesUntitledDerrick kinyaNo ratings yet
- HormonesDocument10 pagesHormonesAmjad AlmousawiNo ratings yet
- Endocrine System CommunicationDocument32 pagesEndocrine System CommunicationalallaallalaNo ratings yet
- Transport of HormonesDocument5 pagesTransport of HormonesJohn MusaNo ratings yet
- 6 Group Phsiology.....Document14 pages6 Group Phsiology.....Ayat ChaudaryNo ratings yet
- Care of Clients With Problem On EndocrineDocument48 pagesCare of Clients With Problem On EndocrineAngel CauilanNo ratings yet
- Biochemistry Assignment Submitted To: Dr. Noureen Submitted byDocument15 pagesBiochemistry Assignment Submitted To: Dr. Noureen Submitted bySalman KhanNo ratings yet
- Endocrinology PDFDocument11 pagesEndocrinology PDFLawrence Genelago GamboaNo ratings yet
- Introduction To Endocrine Physiology: DR EvaDocument17 pagesIntroduction To Endocrine Physiology: DR EvaEmmanuel Julius ChalighaNo ratings yet
- Endocrine PhysiologyDocument41 pagesEndocrine PhysiologyRaj CellaNo ratings yet
- Key Concepts of EndocrinologyDocument131 pagesKey Concepts of Endocrinologykomal pattabiNo ratings yet
- The Endocrine System: Hormone Classification and Mechanisms of Intercellular CommunicationDocument82 pagesThe Endocrine System: Hormone Classification and Mechanisms of Intercellular Communicationعم رNo ratings yet
- Endocrine SystemDocument22 pagesEndocrine Systempranutan739No ratings yet
- Lecture 2 Endocrine SystemDocument53 pagesLecture 2 Endocrine SystemLouella ArtatesNo ratings yet
- ENDOCRINE SYSTEM - Anatomy and PhysiologyDocument10 pagesENDOCRINE SYSTEM - Anatomy and PhysiologyJay Crishnan Morales CajandingNo ratings yet
- Endocrine System: A Tutorial Study GuideFrom EverandEndocrine System: A Tutorial Study GuideRating: 5 out of 5 stars5/5 (1)
- Cesarean SectionDocument36 pagesCesarean SectionHomeground entertainmentNo ratings yet
- Breech PresentationDocument27 pagesBreech PresentationHomeground entertainmentNo ratings yet
- 12558931Document34 pages12558931Homeground entertainmentNo ratings yet
- Cervical CancerDocument87 pagesCervical CancerHomeground entertainmentNo ratings yet
- CaesareanDocument32 pagesCaesareanHomeground entertainmentNo ratings yet
- Perinatal Asphyxia GuideDocument37 pagesPerinatal Asphyxia GuideHomeground entertainmentNo ratings yet
- Amino Acids: Eric Mbindo NjunjuDocument18 pagesAmino Acids: Eric Mbindo NjunjuHomeground entertainmentNo ratings yet
- Biochemistry MBS 230: Eric Mbindo NjunjuDocument20 pagesBiochemistry MBS 230: Eric Mbindo NjunjuHomeground entertainmentNo ratings yet
- Convulsive Disorders: Presenters: Kabwe Chanda EliasDocument30 pagesConvulsive Disorders: Presenters: Kabwe Chanda EliasHomeground entertainmentNo ratings yet
- Drug Treatment of Arrhythmias-Feb 2015Document39 pagesDrug Treatment of Arrhythmias-Feb 2015Homeground entertainmentNo ratings yet
- Proteins: Eric Mbindo NjunjuDocument76 pagesProteins: Eric Mbindo NjunjuHomeground entertainmentNo ratings yet
- Mutations and Their OriginsDocument62 pagesMutations and Their OriginsHomeground entertainmentNo ratings yet
- P6 Disorders of The Basal GangliaDocument12 pagesP6 Disorders of The Basal GangliaHomeground entertainmentNo ratings yet
- Biochemistry MBS 230: Eric Mbindo NjunjuDocument20 pagesBiochemistry MBS 230: Eric Mbindo NjunjuHomeground entertainmentNo ratings yet
- DRUG TREATMENT OF BRONCHIAL ASTHMADocument39 pagesDRUG TREATMENT OF BRONCHIAL ASTHMAHomeground entertainmentNo ratings yet
- Anaphylaxis: Copperbelt University School of Medicine MBCHB Pharmacology February 2015 (DR Sindwa Kanyimba)Document12 pagesAnaphylaxis: Copperbelt University School of Medicine MBCHB Pharmacology February 2015 (DR Sindwa Kanyimba)Homeground entertainmentNo ratings yet
- Principles of Diet Diversity and Dietary Modification - 2Document46 pagesPrinciples of Diet Diversity and Dietary Modification - 2Homeground entertainmentNo ratings yet
- p4 Extra Pyramidal SystemDocument19 pagesp4 Extra Pyramidal SystemHomeground entertainmentNo ratings yet
- p8 The Physiology of The Flocculonodular LobeDocument16 pagesp8 The Physiology of The Flocculonodular LobeHomeground entertainmentNo ratings yet
- p5 Basal GangliaDocument18 pagesp5 Basal GangliaHomeground entertainmentNo ratings yet
- Anti-Tussives-Mucolytics-Expectorants-Feb 2015Document10 pagesAnti-Tussives-Mucolytics-Expectorants-Feb 2015Homeground entertainmentNo ratings yet
- p5 Basal GangliaDocument18 pagesp5 Basal GangliaHomeground entertainmentNo ratings yet
- P9 Disorders of The CerebellumDocument29 pagesP9 Disorders of The CerebellumHomeground entertainmentNo ratings yet
- P3 Pyramidal Tracts of The Somatomotor SystemDocument17 pagesP3 Pyramidal Tracts of The Somatomotor SystemHomeground entertainmentNo ratings yet
- p8 The Physiology of The Flocculonodular LobeDocument16 pagesp8 The Physiology of The Flocculonodular LobeHomeground entertainmentNo ratings yet
- P9 Disorders of The CerebellumDocument29 pagesP9 Disorders of The CerebellumHomeground entertainmentNo ratings yet
- P2 Cortical Areas and Their Role in Contral of MovementDocument18 pagesP2 Cortical Areas and Their Role in Contral of MovementHomeground entertainmentNo ratings yet
- P6 Disorders of The Basal GangliaDocument12 pagesP6 Disorders of The Basal GangliaHomeground entertainmentNo ratings yet
- p4 Extra Pyramidal SystemDocument19 pagesp4 Extra Pyramidal SystemHomeground entertainmentNo ratings yet
- Neurophysiology Systems of the Brain and Spinal CordDocument50 pagesNeurophysiology Systems of the Brain and Spinal CordHomeground entertainmentNo ratings yet
- What Is Mean?: Extrapolation InterpolationDocument2 pagesWhat Is Mean?: Extrapolation InterpolationVinod SharmaNo ratings yet
- Foundations On Expansive Soils: 3.1. BackgroundDocument31 pagesFoundations On Expansive Soils: 3.1. BackgroundbiniNo ratings yet
- Mark Dyczkowski and Trika Journal March 2015 Vol.1.No.1.Document10 pagesMark Dyczkowski and Trika Journal March 2015 Vol.1.No.1.Mark Dyczkoswki and Trika Journal100% (2)
- But Virgil Was Not There": The Lasting Impact of Dante's Homosocial HellDocument7 pagesBut Virgil Was Not There": The Lasting Impact of Dante's Homosocial HellЮлия ЧебанNo ratings yet
- DANGEL 4as LESSON PLANNINGDocument2 pagesDANGEL 4as LESSON PLANNINGCarlz BrianNo ratings yet
- Neolithic Farming Villages Jericho and Catal HuyukDocument1 pageNeolithic Farming Villages Jericho and Catal HuyukPream BoleoNo ratings yet
- Battle Bikes 2.4 PDFDocument56 pagesBattle Bikes 2.4 PDFfranzyland100% (1)
- IM PS Fashion-Business-Digital-Communication-And-Media 3Y Course Pathway MI 04Document7 pagesIM PS Fashion-Business-Digital-Communication-And-Media 3Y Course Pathway MI 04oliwia bujalskaNo ratings yet
- Ffective Riting Kills: Training & Discussion OnDocument37 pagesFfective Riting Kills: Training & Discussion OnKasi ReddyNo ratings yet
- 2011 Mena Annual Reportv1Document73 pages2011 Mena Annual Reportv1Yasmeen LayallieNo ratings yet
- 17a03g - Mosfet - DualDocument5 pages17a03g - Mosfet - DualEletronica01 - BLUEVIXNo ratings yet
- Vocabulary Practice 1Document3 pagesVocabulary Practice 1Phuong AnhNo ratings yet
- The Neuroscience of Autism Spectrum DisordersDocument10 pagesThe Neuroscience of Autism Spectrum DisorderssouciNo ratings yet
- James M. Buchanan - Why I, Too, Am Not A Conservative PDFDocument121 pagesJames M. Buchanan - Why I, Too, Am Not A Conservative PDFHeitor Berbigier Bandas100% (2)
- 1161 Article+Text 5225 1 4 20220712Document9 pages1161 Article+Text 5225 1 4 20220712Warman FatraNo ratings yet
- Samruddhi ComplexDocument7 pagesSamruddhi ComplexNews Side Effects.No ratings yet
- Robotics Process AutomationDocument21 pagesRobotics Process Automationbhaskarkiran.pNo ratings yet
- Mercer Role and Job Analysis InfoDocument3 pagesMercer Role and Job Analysis InfojehaniaNo ratings yet
- MsdsDocument6 pagesMsdsGis GeorgeNo ratings yet
- PW 160-Taliban Fragmentation Fact Fiction and Future-PwDocument28 pagesPW 160-Taliban Fragmentation Fact Fiction and Future-Pwrickyricardo1922No ratings yet
- Basic Load (Individual) Veterinarian Field PackDocument3 pagesBasic Load (Individual) Veterinarian Field PackJohn MillerNo ratings yet
- Ceftriaxone R Salmonella Typhi 02Document8 pagesCeftriaxone R Salmonella Typhi 02docsumitraiNo ratings yet
- ErgonomicsDocument15 pagesErgonomicsdtmNo ratings yet
- 2020 Exam-Sample-Questions-Computer-ScienceDocument8 pages2020 Exam-Sample-Questions-Computer-ScienceNesrine LaradjiNo ratings yet
- Combining Singing and PsycologyDocument6 pagesCombining Singing and PsycologyAna luciaNo ratings yet
- Mikes ResumeDocument2 pagesMikes Resumeapi-312645878No ratings yet
- Four Pillars of EducationDocument42 pagesFour Pillars of EducationWinter BacalsoNo ratings yet
- CBSE Class 10 Science Revision Notes Chapter - 2 Acids, Bases and SaltsDocument11 pagesCBSE Class 10 Science Revision Notes Chapter - 2 Acids, Bases and Saltsmilind dhamaniyaNo ratings yet
- New Balance Case StudyDocument3 pagesNew Balance Case StudyDimas AdityaNo ratings yet
- 5G Antenna Talk TWDocument48 pages5G Antenna Talk TWRohit MathurNo ratings yet