Professional Documents
Culture Documents
Chem 1
Uploaded by
Ezri Mariveles Coda Jr.0 ratings0% found this document useful (0 votes)
8 views9 pagesGas laws relate the pressure, volume, and temperature of a gas. Dalton's law of partial pressures states that the total pressure of a gas mixture is equal to the sum of the individual partial pressures of each gas in the mixture. For example, the total pressure of a mixture of gases A and B is equal to the partial pressure of gas A plus the partial pressure of gas B.
Original Description:
Original Title
ppt-chem-1
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentGas laws relate the pressure, volume, and temperature of a gas. Dalton's law of partial pressures states that the total pressure of a gas mixture is equal to the sum of the individual partial pressures of each gas in the mixture. For example, the total pressure of a mixture of gases A and B is equal to the partial pressure of gas A plus the partial pressure of gas B.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
8 views9 pagesChem 1
Uploaded by
Ezri Mariveles Coda Jr.Gas laws relate the pressure, volume, and temperature of a gas. Dalton's law of partial pressures states that the total pressure of a gas mixture is equal to the sum of the individual partial pressures of each gas in the mixture. For example, the total pressure of a mixture of gases A and B is equal to the partial pressure of gas A plus the partial pressure of gas B.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 9
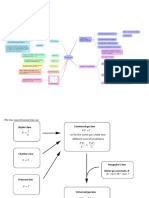
Gas laws
Laws that relate the
pressure, volume,
and temperature of
a gas.
Fatima Coda
Reporter
Dalton’s law of partial pressures is a gas law which states
that the total pressure exerted by a mixture of gases is equal
to the sum of the partial pressures exerted by each
individual gas in the mixture. For example, the total
pressure exerted by a mixture of two gases A and B is equal
to the sum of the individual partial pressures exerted by gas
A and gas B (as illustrated below).
End…
You might also like
- ASD Manual PDFDocument15 pagesASD Manual PDFEzri Mariveles Coda Jr.100% (1)
- Dalton's Law of Partial PressureDocument11 pagesDalton's Law of Partial PressureJohn Eric TajorNo ratings yet
- Chemistry Note The Law of Pression K27Document2 pagesChemistry Note The Law of Pression K27Animation ToysupriseNo ratings yet
- Amagat's LawDocument2 pagesAmagat's LawEn CsakNo ratings yet
- Lecture 2 Thermodynamics-IIDocument11 pagesLecture 2 Thermodynamics-IIMuhammad RashidNo ratings yet
- GASES SendingDocument2 pagesGASES Sendingyoow.youthNo ratings yet
- Chem Assignment 2 (E)Document13 pagesChem Assignment 2 (E)misganamarcos10No ratings yet
- Gas LawDocument3 pagesGas LawJohn Reynard PacsonNo ratings yet
- Dalton's Law Amagat's Law For The Mixture of Real Gases: Whan Woo and Sang Ihn YeoDocument8 pagesDalton's Law Amagat's Law For The Mixture of Real Gases: Whan Woo and Sang Ihn YeoTri SulyonoNo ratings yet
- Gas Laws - Wikipedia PDFDocument17 pagesGas Laws - Wikipedia PDFEmegu MosesNo ratings yet
- Chapter3 IdealgaslawDocument45 pagesChapter3 Idealgaslaw翁绍棠No ratings yet
- Henry's Law Is One of The Gas Laws, Formulated by William Henry in 1803. ItDocument7 pagesHenry's Law Is One of The Gas Laws, Formulated by William Henry in 1803. ItysuntherenNo ratings yet
- The Gas Laws: Cortez Vince Robert Linghon QuishaDocument10 pagesThe Gas Laws: Cortez Vince Robert Linghon QuishaZ ACERNo ratings yet
- Derivation of Dalton's Law of Partial PressureDocument9 pagesDerivation of Dalton's Law of Partial PressureRichard TumbagaNo ratings yet
- Unit 1Document26 pagesUnit 1firehywotNo ratings yet
- Chapter 5 KimiaDocument3 pagesChapter 5 KimiaelmishaenandaeNo ratings yet
- DALTON'SDocument3 pagesDALTON'Sjowelantonio20No ratings yet
- GasesDocument30 pagesGasesWHAT'S SUPNo ratings yet
- What Is GayDocument6 pagesWhat Is GayMiguel LagdameoNo ratings yet
- NB: The 'Handful' Story Is My Own Simplified Way of Understanding This. It's Not Really A ThingDocument2 pagesNB: The 'Handful' Story Is My Own Simplified Way of Understanding This. It's Not Really A ThingMohammed Zaakir AllyNo ratings yet
- Boyle's Law: Chavez, Gabreille R. 1. The Combined Gas LawDocument2 pagesBoyle's Law: Chavez, Gabreille R. 1. The Combined Gas LawGabreille Rullamas ChavezNo ratings yet
- Gas Mixture Lec 2Document18 pagesGas Mixture Lec 2Muhammad Ilyas Qaiser KhanNo ratings yet
- Week 3 PPT AD CHEMDocument8 pagesWeek 3 PPT AD CHEMSophia Ysabelle EstradaNo ratings yet
- Blank LandscapeDocument1 pageBlank LandscapeMira Al-NemratNo ratings yet
- Learning+mtrl+6+gen Chem+2Document10 pagesLearning+mtrl+6+gen Chem+2cappuccino muffinNo ratings yet
- Week 7: Dalton'S Law of Partial PressuresDocument16 pagesWeek 7: Dalton'S Law of Partial PressuresLeonilo Olanda JrNo ratings yet
- Chemistry Act, 1661,: Nationality of Republic of Constitution Act, 1661Document4 pagesChemistry Act, 1661,: Nationality of Republic of Constitution Act, 1661api-515742539No ratings yet
- 2-Chem 1101 The The Properties of Gases & Solutions (Text)Document55 pages2-Chem 1101 The The Properties of Gases & Solutions (Text)Tmmp SmileNo ratings yet
- The Molar Volume of A GasDocument12 pagesThe Molar Volume of A GasabeerNo ratings yet
- فيزياوية 1Document20 pagesفيزياوية 1hagshhsiauhagah516525No ratings yet
- Module 3 Gas LawsDocument12 pagesModule 3 Gas Lawsmarcarvielloydperla3No ratings yet
- Dalton's Law: Chemistry Physics Pressure Partial Pressures Empirical John Dalton Ideal Gas LawsDocument3 pagesDalton's Law: Chemistry Physics Pressure Partial Pressures Empirical John Dalton Ideal Gas LawsJillian BrownNo ratings yet
- Chapter 5 GasesDocument3 pagesChapter 5 GasesKevin HuangNo ratings yet
- Module 5Document16 pagesModule 5Bernard MortilNo ratings yet
- Chemestry Ponderal LawsDocument2 pagesChemestry Ponderal LawsMarinö Chavez100% (1)
- Gas LawsDocument4 pagesGas LawsvanessaNo ratings yet
- Alkali: Latin PH Standard State Acetic Acid Vinegar Sulfuric Acid Car Batteries Redox Oxidation StateDocument4 pagesAlkali: Latin PH Standard State Acetic Acid Vinegar Sulfuric Acid Car Batteries Redox Oxidation StateFatima Erika Ayessa IngkohNo ratings yet
- Study of Gas LawDocument15 pagesStudy of Gas LawKushagra jaiswalNo ratings yet
- Gas Laws: Boyle's LawDocument21 pagesGas Laws: Boyle's LawBobNo ratings yet
- Chapter 1 .Properties of Gases - Lecture 2.Document23 pagesChapter 1 .Properties of Gases - Lecture 2.Mahmoud MahmoudNo ratings yet
- Eos 2Document2 pagesEos 2Tuan NguyenNo ratings yet
- Unit 05 LP09PS - Partial Pressure v06Document12 pagesUnit 05 LP09PS - Partial Pressure v06Steffany FajardoNo ratings yet
- Lec 3Document14 pagesLec 3Not EmeraruduNo ratings yet
- Gas LawsDocument3 pagesGas Lawsqt patootieNo ratings yet
- ChemistryDocument715 pagesChemistryRJ MCNo ratings yet
- Practical ApplicationsDocument3 pagesPractical ApplicationsELvin Jay CasiñoNo ratings yet
- Gas Law: Equation Graph-Draw A Graph Explanation of The LawDocument2 pagesGas Law: Equation Graph-Draw A Graph Explanation of The Lawjesse ParkerNo ratings yet
- Gas LawsDocument2 pagesGas LawsJJAMPPONG PS100% (1)
- Lecture 4 Gas Laws and RelationsDocument28 pagesLecture 4 Gas Laws and RelationsArsal SohrabNo ratings yet
- LEARNING ACTIVITY SHEET-CHEM 1 q1 Week 4Document8 pagesLEARNING ACTIVITY SHEET-CHEM 1 q1 Week 4Jhude JosephNo ratings yet
- Chemistry Picture Vocabulary - Gas LawsDocument23 pagesChemistry Picture Vocabulary - Gas Lawsapi-254514513No ratings yet
- Physical Behavior of Gases: Kinetic TheoryDocument12 pagesPhysical Behavior of Gases: Kinetic TheoryPAUL KOLERENo ratings yet
- Gas LawsDocument3 pagesGas Lawsphebbz_phunky24No ratings yet
- Gaseous States of Matter (HINTS) 2Document2 pagesGaseous States of Matter (HINTS) 2hchawla421No ratings yet
- Chemistry Gas Laws AssignmentDocument6 pagesChemistry Gas Laws AssignmentHans Webster LabordoNo ratings yet
- Gas LawDocument2 pagesGas Lawfranklin.yin2020No ratings yet
- 3 Properties of Ideal GasesDocument52 pages3 Properties of Ideal GasesBERN CERTEZANo ratings yet
- PVT ExperimentDocument23 pagesPVT ExperimentAbdullah FarhanNo ratings yet
- Gas Laws: Pressure, Volume, and Hot AirDocument22 pagesGas Laws: Pressure, Volume, and Hot AirIrwan M. IskoberNo ratings yet
- Assignment: Science IV: 1.) Boyle's LawDocument6 pagesAssignment: Science IV: 1.) Boyle's LawIrish WahidNo ratings yet
- Science 10Document6 pagesScience 10Andy ValdezNo ratings yet
- Sophia - Job Fair 2022Document12 pagesSophia - Job Fair 2022Ezri Mariveles Coda Jr.No ratings yet
- Intelligence and Its Mesurement FinalDocument57 pagesIntelligence and Its Mesurement FinalEzri Mariveles Coda Jr.No ratings yet
- Dev Psych CHAPTER3Document54 pagesDev Psych CHAPTER3Ezri Mariveles Coda Jr.No ratings yet
- Chapter 2Document32 pagesChapter 2Ezri Mariveles Coda Jr.No ratings yet
- Riph 1Document27 pagesRiph 1Ezri Mariveles Coda Jr.No ratings yet
- Acids, Bases, Buffers, PHDocument11 pagesAcids, Bases, Buffers, PHEzri Mariveles Coda Jr.No ratings yet
- "The Effects of Interpersonal Crime On Victims": Reporter: Michel O. Espinosa Bs-Psychology Code: 3175Document6 pages"The Effects of Interpersonal Crime On Victims": Reporter: Michel O. Espinosa Bs-Psychology Code: 3175Ezri Mariveles Coda Jr.No ratings yet
- Self-Assessment Form Mental Health ScaleDocument6 pagesSelf-Assessment Form Mental Health ScaleEzri Mariveles Coda Jr.No ratings yet
- Analysis of The Dog Eater Using A Moralistic Approach To Evaluate The Different Ethical IssuesDocument2 pagesAnalysis of The Dog Eater Using A Moralistic Approach To Evaluate The Different Ethical IssuesEzri Mariveles Coda Jr.No ratings yet
- Divine Healing ServiceDocument22 pagesDivine Healing ServiceEzri Mariveles Coda Jr.No ratings yet
- Mikey's Case (ASD)Document5 pagesMikey's Case (ASD)Ezri Mariveles Coda Jr.No ratings yet