0% found this document useful (0 votes)
826 views14 pagesHard & Soft Acids and Bases: B.Sc. III Year
This document discusses concepts related to hard and soft acids and bases (HSAB) theory including:
- Relative strengths of hydracids can be predicted based on hardness of anions
- Stability of metal complexes can be explained by HSAB (e.g. Cd complexes)
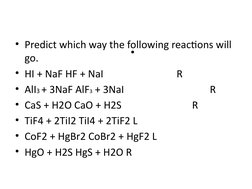
- HSAB can predict the course of reactions (e.g. mercury reactions)
- Qualitative analysis uses HSAB to separate metal cation groups
- Limitations of HSAB theory are that it does not provide strength scales and hardness is not solely dependent on acid/base character
Uploaded by
Gaurav 016Copyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PPTX, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
826 views14 pagesHard & Soft Acids and Bases: B.Sc. III Year
This document discusses concepts related to hard and soft acids and bases (HSAB) theory including:
- Relative strengths of hydracids can be predicted based on hardness of anions
- Stability of metal complexes can be explained by HSAB (e.g. Cd complexes)
- HSAB can predict the course of reactions (e.g. mercury reactions)
- Qualitative analysis uses HSAB to separate metal cation groups
- Limitations of HSAB theory are that it does not provide strength scales and hardness is not solely dependent on acid/base character
Uploaded by
Gaurav 016Copyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PPTX, PDF, TXT or read online on Scribd
- Introduction to HSAB
- Application of HSAB Concepts
- Complex Stability
- Qualitative Analysis
- Separation of Cations
- Ambidentate Bases
- Chemical Reaction Prediction
- Limitations of HSAB Concepts
- Symbiosis


![Application of HSAB Concepts
• Relative stabilities of complexes in Aq
• HSAB explain that [ Cd(CN)4]2- is more stable
than](https://screenshots.scribd.com/Scribd/252_100_85/189/641525528/3.jpeg)