Professional Documents
Culture Documents
Dual Nature of Elements
Uploaded by
Tarnate Tacay100%(1)100% found this document useful (1 vote)
18 views27 pagesCopyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
100%(1)100% found this document useful (1 vote)
18 views27 pagesDual Nature of Elements
Uploaded by
Tarnate TacayCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 27
REVIEW
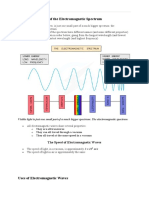
Electromagnetic radiation may be
visible or invisible depending on their
wavelength. The human eye can
detect the light spectrum ranging
from 400 nanometers (violet) to about
700 nanometers (red). Outside this
wavelength range, light is invisible.
REVIEW
REFLECTION is the bouncing
off of light as it strikes a
surface.
REFRACTION is the bending
of light as it passes from one
REVIEW
The INTERFERENCE of light is
the phenomenon of multiple light
waves interacting with one another
under certain circumstances,
causing the combined amplitudes
of the waves to either increase or
decrease.
REVIEW
DIFFRACTION of light is
defined as the bending of
light around corners such
that it spreads out and
illuminates areas where a
shadow is expected.
REVIEW
Polarization, in Physics, is
defined as a phenomenon
caused due to the wave
nature of electromagnetic
radiation.
REVIEW
Christian Huygens proposed
the Wave Theory of Light in
1678 in which light is made
up of waves.
REVIEW
Thomas Young studied the
interference of light waves
using the double-slit
experiment in 1803.
REVIEW
Albert Einstein proposed
light is composed of tiny
particles called photons.
REVIEW
Since red light has the least
frequency in the color spectrum of
light, it also has the least amount of
energy and can therefore be used in
photographic dark rooms because
it would have the least effect on a
very sensitive paper film.
REVIEW
Given that ultraviolet has a higher
frequency than the visible light, it
follows that it would also have
greater energy, which causes us to
get easily sunburned under the UV
light compared to visible light.
DUAL
NATURE
OF
ELECTRONS
PARTICLE
A minute portion of matter and
the smallest known building
blocks of the universe.
A small finite object that you can
hold in your hand.
Have momentum and their
positions can be identified.
GEORGE JOHNSTONE
STONEY
Coined the term electron as
an electric charge quantity
in 1894.
JOSEPH JOHN THOMSON
Identified electron as a
particle using cathode ray
tubes.
RUTHERFORD’S ATOMIC
MODEL
ELECTRON is a
negatively-
charged
subatomic
particle and has
no known
Electrons are
Particle.
Is it a wave?
WAVE
A disturbance that travels
through space-time and medium
by transferring energy from one
place to another.
The wave medium transports the
wave from its source to other
locations.
WAVE
CONSTRUCTIVE INTERFERENCE
When waves meet from crests to
crests and trough to trough, then
the displacement is reinforced.
CONSTRUCTIVE INTERFERENCE
DESTRUCTIVE INTERFERENCE
When waves meet from crests to
troughs, then the displacement is
cancelled.
DESTRUCTIVE INTERFERENCE
LOUIS DE BROGLIE
Italian physicist who postulated
that if light waves also exhibit
particle behavior, then a particle
should also exhibit wavelike
behavior.
LOUIS DE BROGLIE
He also theorized that the
wavelength of a particle is related
to Planck’s constant and inversely
proportional to its momentum.
DE BROGLIE
WAVELEGNTH
Where:
= de Broglie’s wavelength of a particle
h = Planck’s constant (
m = mass
v = velocity
CLINTON DAVISSON AND
LESTER GERMER
American physicist who tested
that scattered electrons will
appear from all directions with
little dependence on their
intensity, scattering angle, and
energy of the primary beam.
You might also like
- Propagation of Light: Physical Science - Week 3ADocument28 pagesPropagation of Light: Physical Science - Week 3AJen JvsjvsNo ratings yet
- Q2 Week 3Document58 pagesQ2 Week 3Zayra Melgar AtreroNo ratings yet
- The Nature of Light PDFDocument15 pagesThe Nature of Light PDFthinaNo ratings yet
- ORGANIZATIONAL LEADERSHIP - Tañedo, Maria Niña GuiamDocument28 pagesORGANIZATIONAL LEADERSHIP - Tañedo, Maria Niña GuiamMaria Nina TanedoNo ratings yet
- Mendelian GeneticsDocument28 pagesMendelian GeneticsRea AnsusNo ratings yet
- Quarter 4: Week 3 Properties of Light: Mr. Ronald P. CoricorDocument52 pagesQuarter 4: Week 3 Properties of Light: Mr. Ronald P. Coricor•MALCOLM X•No ratings yet
- G11 Propagation of LightDocument32 pagesG11 Propagation of LightRyan Dave MacariayNo ratings yet
- WorksheetHandoutNo.13 MELVIN GRAFILDocument15 pagesWorksheetHandoutNo.13 MELVIN GRAFILDulce J. LuatonNo ratings yet
- JHS Physics - BOW - MELC PDFDocument12 pagesJHS Physics - BOW - MELC PDFJohnry Guzon ColmenaresNo ratings yet
- Science 8''may31 FinalDocument83 pagesScience 8''may31 FinalPrincess Ronquillo - DuqueNo ratings yet
- Physical Science Quarter 3 Week 2: Not For SaleDocument7 pagesPhysical Science Quarter 3 Week 2: Not For SaleChristien Kate GonzalesNo ratings yet
- Section 5.1 Newton's First Law of MotionDocument36 pagesSection 5.1 Newton's First Law of Motiontwy113No ratings yet
- How Representative Animals ReproduceDocument15 pagesHow Representative Animals ReproduceRubyrose Nieves100% (1)
- Life Science Module 4 Lesson 3 and 4Document76 pagesLife Science Module 4 Lesson 3 and 4Mae AlfaroNo ratings yet
- Lesson 3 Intermolecular ForcesDocument13 pagesLesson 3 Intermolecular ForcesChristine SenaNo ratings yet
- Physical Sciences: Concept of Atomic Number That Led To The Synthesis of New Elements in The LaboratoryDocument40 pagesPhysical Sciences: Concept of Atomic Number That Led To The Synthesis of New Elements in The LaboratoryMaria Rosabel Ilagan100% (1)
- Week-2-Q1-Gen Chem-Sep-4-8-DllDocument15 pagesWeek-2-Q1-Gen Chem-Sep-4-8-DllJennette BelliotNo ratings yet
- Introduction To ReproductionDocument45 pagesIntroduction To ReproductionCharmaine Fernandez100% (1)
- Shs-Daily-Lesson-in-physical Science JMG Q3W1Document14 pagesShs-Daily-Lesson-in-physical Science JMG Q3W1Joseph GutierrezNo ratings yet
- Q4 M1 Ancient GreeksDocument65 pagesQ4 M1 Ancient GreeksKeziah ChristineNo ratings yet
- I. EngageDocument2 pagesI. Engagefaith cayaNo ratings yet
- Science Dept Lesson Plan For Grade 12-PhysicsDocument20 pagesScience Dept Lesson Plan For Grade 12-Physicschandra boseNo ratings yet
- Elements and CompoundsDocument8 pagesElements and CompoundsJerry De Leon TaayNo ratings yet
- Physical-Science-Module 5 Polarity and Intermolecular Forces of AttractionDocument45 pagesPhysical-Science-Module 5 Polarity and Intermolecular Forces of AttractionJoana CastilloNo ratings yet
- Els LPDocument7 pagesEls LPRizeh Hermie Grace PunayNo ratings yet
- One Punch ReviewerDocument5 pagesOne Punch ReviewerMorelei FernandezNo ratings yet
- Unifying Themes in The Study of Life ScienceDocument41 pagesUnifying Themes in The Study of Life ScienceKath Tan AlcantaraNo ratings yet
- Lesson Plan Name of School Class / Semester Subject CurriculumDocument7 pagesLesson Plan Name of School Class / Semester Subject CurriculumNurul JanahNo ratings yet
- WEEK 3 - Q2 - Earth and LifeDocument22 pagesWEEK 3 - Q2 - Earth and Lifenoreen lubindino100% (1)
- Week-4-Q1-Gen Chem-Sep-18-22-DllDocument11 pagesWeek-4-Q1-Gen Chem-Sep-18-22-DllJennette BelliotNo ratings yet
- PS - Quarter 2 - Week 1Document6 pagesPS - Quarter 2 - Week 1edgardo viadorNo ratings yet
- Week 1 - Digestive SystemDocument26 pagesWeek 1 - Digestive Systemlau dashNo ratings yet
- Stellar EvolutionDocument21 pagesStellar EvolutionJohn Reign Matanguihan100% (1)
- Anticipation-Reaction Guide Put If AGREE To The Statement and If You DISAGREEDocument1 pageAnticipation-Reaction Guide Put If AGREE To The Statement and If You DISAGREEYongco MarloNo ratings yet
- Breathe In: Write Your Answer On These Activity SheetDocument4 pagesBreathe In: Write Your Answer On These Activity Sheetirah jane valentinoNo ratings yet
- Do Now:: What Is Motion? Describe The Motion of An ObjectDocument22 pagesDo Now:: What Is Motion? Describe The Motion of An ObjectJoan Melendres100% (1)
- LP Law of Reflection - Myrafe RodellasDocument8 pagesLP Law of Reflection - Myrafe Rodellasmyrafe rodellasNo ratings yet
- HeatDocument23 pagesHeatNinja ni BUHAY BUKID MIX VLOGNo ratings yet
- The Nature and Properties of Light PDFDocument32 pagesThe Nature and Properties of Light PDFtees cNo ratings yet
- Science 8-Quarter 2-Lesson 1Document20 pagesScience 8-Quarter 2-Lesson 1Ms. Rhonabelle Llerin PagudNo ratings yet
- Modeling Lesson OsmosisDocument4 pagesModeling Lesson Osmosisapi-263275919No ratings yet
- COT1Document13 pagesCOT1Margei CutadNo ratings yet
- Grade 9 Science Lesson Plan Second Quarter by Welfredo Yu JRDocument12 pagesGrade 9 Science Lesson Plan Second Quarter by Welfredo Yu JRWelfredo Red Wolf YuLptNo ratings yet
- Charging by InductionDocument7 pagesCharging by InductionShikhar Virmani100% (1)
- Earth and Life Science Lesson 4-Genetic EngineeringDocument23 pagesEarth and Life Science Lesson 4-Genetic Engineeringjhondee lagramaNo ratings yet
- Unifying Themes of LifeDocument33 pagesUnifying Themes of LifeRyan Dave MacariayNo ratings yet
- 10 Third Second NCR-Quezon City CD6 Science 10: I. ObjectivesDocument3 pages10 Third Second NCR-Quezon City CD6 Science 10: I. ObjectivesHeidi Balabbo100% (1)
- ReproductionDocument37 pagesReproductionChristian Quiñones100% (1)
- Light Lesson PlanDocument8 pagesLight Lesson PlanObul Reddy PolimeraNo ratings yet
- Canarvacanan National High SchoolDocument3 pagesCanarvacanan National High SchoolMichael Ervin GuerzonNo ratings yet
- 1.1 Electricity in Materials: Session 01 Question and AnswersDocument9 pages1.1 Electricity in Materials: Session 01 Question and AnswersPassPhysicsSym100% (1)
- Physical Science DLL (Week7)Document6 pagesPhysical Science DLL (Week7)jessiry lascanoNo ratings yet
- DLL Earth&Life Cot1Document2 pagesDLL Earth&Life Cot1Cindy ManipolNo ratings yet
- Ppt-Sci 2Document15 pagesPpt-Sci 2klichedoNo ratings yet
- Physical Science IntroductionDocument16 pagesPhysical Science IntroductionAngekyla BarroquilloNo ratings yet
- Physical Science - q3 - Slm5Document15 pagesPhysical Science - q3 - Slm5John PaulNo ratings yet
- Planet Earth and Earth's Subsystem PresentationDocument14 pagesPlanet Earth and Earth's Subsystem PresentationMarra Melmarie MalacaoNo ratings yet
- Polar Non PolarDocument54 pagesPolar Non PolarJhon Gabriele CuramengNo ratings yet
- OpticsDocument35 pagesOpticsMd. Sayeed HasanNo ratings yet
- Prism OpticsDocument32 pagesPrism OpticsFredNo ratings yet
- Outdoor Directional Tri-Band Antenna: ODV-065R15B15J15JDocument1 pageOutdoor Directional Tri-Band Antenna: ODV-065R15B15J15JClaudia Mendes100% (1)
- 9-Dental Radiation PhysicsDocument27 pages9-Dental Radiation PhysicsAbdullah El-ZaaboutNo ratings yet
- Lecture 3.the Energetic Grid of The Earth: What Is The Electromagnetic Spectrum?Document10 pagesLecture 3.the Energetic Grid of The Earth: What Is The Electromagnetic Spectrum?Dr. Shashank RkNo ratings yet
- Antenas RVRDocument140 pagesAntenas RVReleazarNo ratings yet
- Refractive Index of Kerocene OilDocument10 pagesRefractive Index of Kerocene OilchethanNo ratings yet
- 3.3 Electromagnetic SpectrumDocument4 pages3.3 Electromagnetic SpectrumDhanBahadurNo ratings yet
- Microwave TransmissionDocument18 pagesMicrowave TransmissionMarilyn Castro LaquindanumNo ratings yet
- The Nature of Light: B.Sc. PhysicsDocument6 pagesThe Nature of Light: B.Sc. PhysicsGhufran MuayadNo ratings yet
- Thin Film Calculator From Dissertation - JunZhang - 080110 - Optimized PDFDocument26 pagesThin Film Calculator From Dissertation - JunZhang - 080110 - Optimized PDFAlvaroFernandezVillarNo ratings yet
- Alpha DecayDocument3 pagesAlpha DecayRiyan HidayatNo ratings yet
- DSLR SpectrometerDocument16 pagesDSLR SpectrometerematlisNo ratings yet
- Zoink Answer Questions: Wave TestDocument6 pagesZoink Answer Questions: Wave TestChachi SeonsaengNo ratings yet
- Nature of LightDocument4 pagesNature of Lightalleaheunice29No ratings yet
- A) Title of Experiment: D) TheoryDocument7 pagesA) Title of Experiment: D) TheoryAtif BaigNo ratings yet
- A Short Introduction To Free Electron Lasers: The Cern Accelerator SchoolDocument45 pagesA Short Introduction To Free Electron Lasers: The Cern Accelerator SchoolHorenhop DolakovNo ratings yet
- Visible LightDocument26 pagesVisible LightDotty dottyNo ratings yet
- Antenna JournalsDocument1 pageAntenna JournalsNur FaiqahNo ratings yet
- Principles of Computed Radiography: Group 1Document18 pagesPrinciples of Computed Radiography: Group 1Kurt Izen OrtegaNo ratings yet
- PhotoelasticityDocument41 pagesPhotoelasticityKapil SaraswatNo ratings yet
- New Minimum Requirement Questions For Biophysics PDFDocument14 pagesNew Minimum Requirement Questions For Biophysics PDFLydia AitNo ratings yet
- Task 2 - ValentinaMilord PDFDocument13 pagesTask 2 - ValentinaMilord PDFwendy lucrecia vasquez tijaro100% (3)
- Lakshminarayan Hazra - Foundations of Optical System Analysis and Design-CRC Press (2021)Document775 pagesLakshminarayan Hazra - Foundations of Optical System Analysis and Design-CRC Press (2021)nl.chandrasekarNo ratings yet
- Kohl Surma Galena CollyriumDocument5 pagesKohl Surma Galena CollyriumjivasumanaNo ratings yet
- DR Mohamed Optics Subject AssigmentDocument3 pagesDR Mohamed Optics Subject AssigmentEyasu Kanbato OtoroNo ratings yet
- LQ Grade 7Document2 pagesLQ Grade 7Irene MartinNo ratings yet
- Ioana Presentation PDFDocument10 pagesIoana Presentation PDFazairapolaNo ratings yet
- MirageDocument1 pageMirageHerru IndrawanNo ratings yet
- Heavy Petting: by Miles MathisDocument7 pagesHeavy Petting: by Miles MathisSlava OlchevskiNo ratings yet
- Massachusetts Institute of Technology Department of Electrical Engineering and Computer ScienceDocument3 pagesMassachusetts Institute of Technology Department of Electrical Engineering and Computer ScienceKrishna GNo ratings yet